Olfactory Dysfunction Discriminates Probable Alzheimer's Dementia From Major Depression
Abstract
The present study was conducted to cross-validate and extend the hypothesis that olfactory dysfunction could discriminate between groups of patients with Alzheimer's disease and major depression. Forty patients meeting the DSM-IV criteria for either Alzheimer's disease or major depression (20 per group) underwent assessment with the Pocket Smell Test (PST), a three-item screening measure of odor identification, and the Mini-Mental State Examination (MMSE). A PST score of ≤1 (1 or 0 correct) discriminated between the groups with a hit rate of 97.5% (sensitivity=95%, specificity=100%). The optimal hit rate for the MMSE (≤24) was less effective (hit rate=90%, sensitivity=80%, specificity=100%). Age, gender, and education had minimal impact on the PST for both groups. Olfactory assessment continues to add to the diagnostic utility in the differential diagnosis of Alzheimer's disease versus major depression in elderly patients.
The loss of sense of smell is an often-underutilized indicator of brain pathology. Olfactory dysfunction, for example, has been noted in a number of neuropsychiatric conditions, including Alzheimer's disease (AD),1–5 Parkinson's disease,6 human immunodeficiency virus–type 1 (HIV),7 motor neuron disease,8 schizophrenia,4 and advanced anorexia.9 With the exception of one study,10 it has been reported that both healthy2,4,5 and depressed adults11,12,13 and elders3 exhibit intact odor identification skills. Recently, the assessment of olfactory functioning has been used to discriminate between patient and nonpatient samples. For example, a number of investigators have reported odor identification differences in AD patients and elderly control subjects,2,4,5 and these differences may prove useful at the diagnostic level. Of potential diagnostic utility for neuropsychiatrists and neuropsychologists would be a comparison between elderly patients with AD and elderly patients with major depression.
In the only investigation of this particular comparison to date, Solomon et al.3 administered the Pocket Smell Test (PST), a screening measure of odor identification derived from the University of Pennsylvania Smell Identification Test14 (UPSIT), to a group of patients with AD and a group of patients with major depression. On this three-item test, a cutoff score of two or more errors correctly classified 90% of the sample, with the AD patients being more impaired than the depressed patients. Despite the clinically significant and diagnostically useful findings of this study, the authors note two limitations. First, there was no assessment of the subjects' cognitive functioning, and second, the effects of demographic variables (e.g., age, education, gender) were not fully examined. The potential influence of demographic variables has not been assessed systematically in patients undergoing concomitant cognitive and odor identification testing. Although it is well known that age and education have significant correlations with scores on the Mini-Mental State Examination15 (MMSE), less is known about the effects of demographic variables on odor identification test performance in patient samples. Doty16 has shown age and, to a lesser extent, gender to be related to odor identification scores on the UPSIT. No data exist regarding the possible effects of demographic variables on PST performance.
Further, study of the relationship between odor identification performance and MMSE scores has yielded mixed results. For example, Serby et al.5 assessed UPSIT and MMSE performances in 55 AD patients and 57 control subjects and found a statistically significant correlation of 0.48 between the measures. Similarly, Larsson et al.17 reported a correlation of 0.62 between MMSE scores and a 20-item odor identification task in 11 patients with AD and 11 matched control subjects. Conversely, Moberg et al.18 found no statistically significant correlation between MMSE scores and UPSIT performance in 20 patients with AD. The present study was conducted in an attempt to cross-validate the findings of Solomon et al.,3 to extend the previous PST work by including a cognitive assessment (MMSE) of the subjects, and to evaluate the effects of demographic variables on MMSE and PST performance.
METHODS
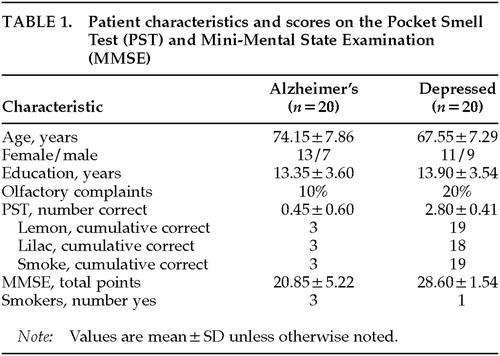
Patients were 55 years of age or older, met DSM-IV criteria for a diagnosis of either Alzheimer's disease or major depression, and gave informed consent. Diagnoses had been established by board-certified (adult and/or geriatric) psychiatrists, neurologists, or neuropsychologists who had the opportunity to follow these patients longitudinally. Patients were excluded if they had a history of neurologic, psychiatric, or medical disorder that could affect olfaction adversely (e.g., traumatic brain injury, schizophrenia, Parkinson's disease, HIV-positive status, upper respiratory illness). Demographic characteristics of the patient groups are presented in Table 1. Patients were also questioned about whether they had experienced any recent change in sense of smell (“olfactory complaints,” Table 1).
All patients were evaluated with the Pocket Smell Test (PST)19 and the MMSE.20 The PST is a three-item microencapsulated “scratch-and-sniff” measure derived from the University of Pennsylvania Smell Identification Test.14 On each item, the odor is released and the patient chooses one of the four response alternatives. The response alternatives were read to the patient continuously until a response was made. Patients were encouraged to guess if they were unsure. Correct responses were lemon, lilac, and smoke, as in the previous study by Solomon et al.3 The PST has been shown to be sensitive to olfactory deficits in Alzheimer's disease.1 The MMSE is a widely used screening measure of cognitive functioning that taps orientation, attention, short-term memory, and language abilities. With a maximum score of 30 and an “impairment” cutoff score of approximately 23, the MMSE has been shown to be sensitive to the cognitive deficits in Alzheimer's.
RESULTS
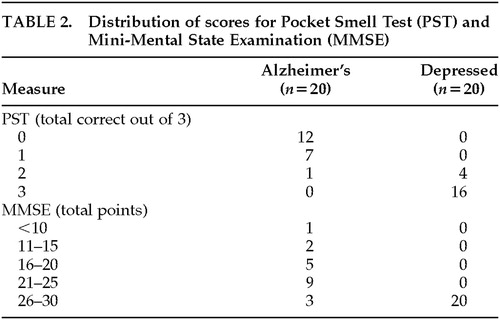
The distribution of PST and MMSE scores is presented in Table 2. The Alzheimer's group, which was older than the depressed group (P=0.009), performed significantly worse than the depressed group on the PST (t=14.38, df=38, P<0.001). The Alzheimer's group, which did not differ from the depressed group in years of education (P=0.629), also performed significantly worse than the depressed group on the MMSE (t=6.37, df=38, P<0.001). The correlations between demographic variables and PST and MMSE scores for each group are presented in Table 3.
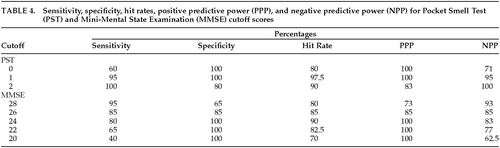
The top part of Table 4 lists the sensitivity, specificity, hit rate, positive predictive power, and negative predictive power for various cutoff scores on the PST. There was only one false negative in the Alzheimer's group (a 69-year-old female who guessed 2/3 correct on the PST and had an MMSE score of 20/30). The bottom part of Table 4 lists the sensitivity, specificity, hit rate, positive predictive power, and negative predictive power for various cutoff scores on the MMSE. There were four false negatives in the Alzheimer's group, with all having PST scores of 1 or 0 correct. The three highest MMSE scores (one of 29 and two of 28) were all for patients who had either a master's or doctoral-level education but scored ≤1 correct on the PST.
DISCUSSION
The results of the present study support the use of the PST in differentiating patients with AD from patients with major depression. As in earlier works,1–5 significant deficits in olfaction were observed in the AD group but not in the major depression group. The present study also replicates the findings of Solomon et al.3 in that a cutoff score of two or more errors correctly classified 97.5% (all but one) of the AD and depressed subjects. The results further indicate that the PST was more accurate in classifying the two groups than was the MMSE, a standard in the field for brief cognitive assessment and diagnosis of dementia.
Odor identification testing is sensitive to AD because the entorhinal cortex (a major component of the olfactory system) is the first brain area affected by neurofibrillary tangles,21,22 one of the defining neuropathological markers of AD. To date, there is no neuropathological evidence of entorhinal cortex or other olfactory-system damage in major depression. Hence, odor identification testing can serve as an empirical discriminator between the two conditions.
The effects of age, gender, and education showed minimal impact on the PST for both the Alzheimer's and depression groups, as no correlations were statistically significant. The findings suggest that these demographic variables do not systematically affect PST performances. However, significant correlations were noted between age and MMSE scores in the AD group, and between gender and MMSE scores in the depression group (for both analyses, r=0.58, P<0.05). These correlations indicate that age accounted for approximately 34% of the variance in MMSE scores for the AD group and that gender accounted for about the same amount of variance in the depressed group. Gender differences in the MMSE scores in the depressed group (with males scoring 1.7 points higher) were likely confounded by gender differences within this group on age and education variables (depressed males were younger and slightly more educated than depressed females). Therefore, interpretations of MMSE scores are not as straightforward as PST scores.
The correlation between PST and MMSE scores was weakly positive (but nonsignificant) for the AD group and virtually nil for the depression group. These results are consistent with the findings of Moberg et al.,18 who found no statistically significant correlation between odor identification and MMSE performance in AD patients. An analysis of studies reporting a positive correlation between odor identification and MMSE scores5,17 reveals that AD patients and control subjects were combined in the statistical analysis. Indeed, Morgan et al.23 found a correlation of 0.73 between UPSIT and MMSE scores in normal control subjects but no significant correlation for AD patients. The inconsistencies in published reports of the relationship between odor identification and MMSE scores may be a function of combining experimental groups in the correlational analyses. Future studies might endeavor to separate the groups when assessing this relationship.
The potential impact of medication and smoking on odor identification skills has been addressed in various patient populations, with few definitive findings noted. Ship et al.24 found no relationship between medication use and scores on the UPSIT in generally healthy, community-dwelling, nondemented elders. Neuroleptic use in patients with schizophrenia has been shown to be unrelated to odor identification skills.25 Kesslak et al.26 reported no relationship between UPSIT scores and medication states in patients with AD. Gross-Isseroff et al.27 found improved olfactory sensitivity to isoamyl acetate in depressed patients 6 weeks after initiation of antidepressant pharmacotherapy. No significant relationship between smoking and UPSIT scores has been reported in patients with HIV infection,7 schizophrenia,25,28 or various other neurological disorders.18 Smoking has been noted to be unrelated to odor sensitivity capacity in patients with major depression and obsessive-compulsive disorder.27 Within the present study, no significant correlations were observed between smoking status and either total PST score or any of the individual PST items for either the AD or the major depression group. Future studies should include smoking and medication variables to further assess these possible relationships.
As noted in Solomon et al.,3 and as replicated in this study, only 10% of the AD patients and 15% to 20% of the depressed patients reported any awareness of olfactory decline. Olfactory testing (PST) indicated objective evidence of odor identification deficits (i.e., PST score of <3 correct) in 100% of the AD patients and 20% of the depressed patients. Patient report of olfactory change is therefore considered unreliable, and formal testing is warranted.
Despite the methodological and statistical extension of Solomon et al.,3 a number of other limitations of the present study should be noted. First, the cognitive evaluation of the patients used in the study was limited to a screening device, and a more thorough neuropsychological evaluation would likely yield more impressive diagnostic utility and accuracy. A second shortcoming was the lack of a more objective description of the group with major depression. A Hamilton Depression Rating Scale or Beck Depression Inventory score would have been useful, and future studies should include such measures. This study does not support the use of the PST as the sole indicator in the differential diagnosis of AD versus major depression. Rather, it is intended to supplement MMSE (or other cognitive test scores) and assist the clinician in more successfully directing the patient's care. Future investigations relating the PST to other neuropsychological measures are warranted. A further limitation relates to the age difference between the two patient groups. Although the AD group was older than the depressed group, this difference of 6.6 years appears clinically inconsequential in light of the relationship between age and PST performance. Further, the age variable accounted for less than 7% of the variance in AD patients' PST scores, a finding similar to the less than 5% variance noted in Solomon et al.3 Nonetheless, future studies should endeavor to have groups with no age differences. Again, we must emphasize that all patients in the AD group are cases of Probable AD; autopsy confirmation is lacking. Any misdiagnoses could certainly alter the results of this study. Finally, although the present study explored the relationship between olfactory and cognitive functioning, comparisons of the PST with other types of functioning that are also impaired in AD patients may be of additional value to the clinician. Assessments of mood, activities of daily living, and physical functioning and their relationship to olfactory functioning may further delineate AD groups from non-AD groups.
It should be noted that despite these encouraging results, the PST has not yet faced the experimental challenge of discriminating clinical cases of depressive pseudodementia from AD in practice. In this study and in the preceding one,3 the PST has been used to differentiate established cases of AD from major depression. This procedure, however, is consistent with the standard method for developing a diagnostic test in medicine, which is to see how well the test discriminates between clear-cut instances of two potentially similar conditions. The initial study indicated the potential utility of the PST, and the current study cross-validated and extended the findings. The next step, of course, would be to test the PST with clinical cases in which patients present with mixed affective and cognitive symptoms.
The results of the present study provide primary care physicians, neurologists, neuropsychiatrists, and neuropsychologists with a helpful diagnostic indicator in the differential diagnosis of AD versus major depression. The PST is a brief, portable, inexpensive, and user-friendly measure that has been used successfully to discriminate between these two groups, which often present with similar clinical pictures. Effects of age, gender, and education on the PST (unlike the MMSE) are negligible for both of these patient groups. The assessment of olfactory functioning continues to provide valuable information for the clinician in the differential diagnosis of AD versus major depression and subsequent treatment interventions.
ACKNOWLEDGMENTS
Support for this research came in part from an unrestricted research grant from Pfizer Pharmaceutical and Eisai, Inc.
 |
 |
 |
 |
1 Solomon GS: Anosmia in Alzheimer disease. Percept Mot Skills 1994; 79:1249–1250Google Scholar
2 Mesholam RI, Moberg PJ, Mahr RN, et al: Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol 1998; 55:84–90Crossref, Medline, Google Scholar
3 Solomon GS, Petrie WM, Hart JR, et al: Olfactory dysfunction discriminates Alzheimer's dementia from major depression. J Neuropsychiatry Clin Neurosci 1998; 10:64–67Link, Google Scholar
4 Moberg PJ, Doty RL, Mahr RN, et al: Olfactory identification in elderly schizophrenia and Alzheimer's disease. Neurobiol Aging 1997; 18:163–167Crossref, Medline, Google Scholar
5 Serby M, Larson P, Kalkstein DS: The nature and course of olfactory deficits in Alzheimer's disease. Am J Psychiatry 1991; 148:357–360Crossref, Medline, Google Scholar
6 Hawkes CH, Shepard BC, Daniel SE: Olfactory dysfunction in Parkinson's disease. J Neurol Neurosurg Psychiatry 1997; 62:436–446Crossref, Medline, Google Scholar
7 Westervelt HJ, McCaffrey RJ, Cousins JP, et al: Longitudinal analysis of olfactory deficits in HIV infection. Archives of Clinical Neuropsychology 1997; 12:557–565Crossref, Medline, Google Scholar
8 Elian M: Olfactory impairment in motor neuron disease: a pilot study. J Neurol Neurosurg Psychiatry 1991; 54:927–928Crossref, Medline, Google Scholar
9 Fedoroff I, Stoner SA, Andersen, AE, et al: Olfactory dysfunction in anorexia and bulimia nervosa. Int J Eat Disord 1995; 18:71–77Crossref, Medline, Google Scholar
10 Serby M, Larson P, Kalkstein D: Olfactory sense in psychoses. Biol Psychiatry 1990; 28:829–830Medline, Google Scholar
11 Amsterdam JD, Settle RG, Doty RL, et al: Taste and smell perception in depression. Biol Psychiatry 1987; 22:1477–1481Google Scholar
12 Kopala LC, Clark C, Hurwitz T: Olfactory deficits in neuroleptic-naive patients with schizophrenia. Schizophr Res 1992: 8:245–250Google Scholar
13 Warner M, Peabody C, Cserninsky J: Olfactory functioning in schizophrenia and depression. Biol Psychiatry 1990; 27:457–467Crossref, Medline, Google Scholar
14 Sensonics, Inc.: The Smell Identification Test. Haddon Heights, NJ, Sensonics, Inc. (n.d.)Google Scholar
15 Crum RM, Anthony JC, Bessett SS, et al: Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993; 269:2386–2391Google Scholar
16 Doty RL: The Smell Identification Test Administration Manual, 3rd edition. Haddon Heights, NJ, Sensonics, Inc., 1995Google Scholar
17 Larsson M, Semb H, Winblad B, et al: Odor identification in normal aging and early Alzheimer's disease. Neuropsychology 1999; 13:47–53Crossref, Medline, Google Scholar
18 Moberg P, Doty RL, Turetsky BI, et al: Olfactory identification deficits in schizophrenia: correlation with duration of illness. Am J Psychiatry 1997; 154:1016–1018Google Scholar
19 Sensonics, Inc.: The Pocket Smell Test. Haddon Heights, NJ, Sensonics, Inc. (n.d.)Google Scholar
20 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
21 Braak H, Braak E: Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica (Berlin) 1991; 82:239–259Crossref, Medline, Google Scholar
22 Pearson RC, Esiri MM, Hiorns RW, et al: Anatomical correlates of the distribution of the pathologic changes in the neocortex in Alzheimer's disease. Proc Natl Acad Sci USA 1985; 82:4531–4534Google Scholar
23 Morgan CD, Nordin S, Murphy C: Odor identification as an early marker for Alzheimer's disease: impact of lexical functioning and detection sensitivity. J Clin Exp Neuropsychol 1995; 17:793–803Crossref, Medline, Google Scholar
24 Ship JA, Pearson JD, Cruise LJ, et al: Longitudinal changes in smell identification. J Gerontol A Biol Sci Med Sci 1996; 51A:M86–M91Google Scholar
25 Kopak L, Good K, Honer WG: Olfactory hallucinations and olfactory identification ability in patients with schizophrenia and other psychiatric disorders. Schizophr Res 1994; 12:205–211Crossref, Medline, Google Scholar
26 Kesslak JP, Cotman CW, Chui HC, et al: Olfactory tests as possible probes for detecting and monitoring Alzheimer's disease. Neurobiol Aging 1988; 9:399–403Crossref, Medline, Google Scholar
27 Gross-Isseroff R, Luca-Haimovia K, Sasson Y, et al: Olfactory sensitivity in major depressive disorder and obsessive compulsive disorder. Biol Psychiatry 1994; 35:798–802Crossref, Medline, Google Scholar
28 Houlihan DJ, Flaum M, Arnold SE: Further evidence for olfactory identification deficits in schizophrenia. Schizophr Res 1994; 12:179–182Crossref, Medline, Google Scholar



