Treatment of Dementia-Associated Agitation With Gabapentin
Abstract
The authors describe the use of gabapentin in the treatment of 4 outpatients with dementia-associated agitation. On the basis of clinical case reports and the Overt Agitation Severity Scale, all 4 patients had reduced agitation with gabapentin. Three of 4 patients were successfully titrated to a full dose of 2,400mg/day. These findings suggest a possible role for gabapentin in the behavioral management of patients with dementia.
Patients with dementia experience progressive cognitive decline and may manifest a variety of behavioral symptoms. These symptoms, which include agitation, psychosis, screaming, wandering, insomnia, anxiety, and depression, are often extremely difficult for caregivers to manage1 and are a common cause of placement into nursing homes.2
Agitation in elderly persons has been defined as “inappropriate verbal, vocal, or motor activity that is not explained by needs or confusion per se.”3 Agitation has been reported to occur in outpatients with Alzheimer's disease at a frequency of 24% by Teri et al.4 and 48% by Reisberg et al.5 The latter group found that violent agitation occurred in 30% of patients.
The psychopharmacologic treatment of agitation in dementia had previously centered on the use of antipsychotic medications such as haloperidol and thioridazine. Schneider et al.6 performed a meta-analysis of controlled studies of antipsychotics. Although the authors found that dementia patients with agitation responded significantly better to medication than to placebo, they also found the benefits of medication to be “modest.” Difficulties caused by the use of antipsychotics include restlessness, which can worsen agitation,7 and tardive dyskinesia, which has a higher incidence in elderly patients.7 In one study with risperidone, an atypical antipsychotic, 50% of patients exhibited extrapyramidal side effects.8
As a result of the drawbacks of antipsychotic treatment, other agents have been applied to the problem of dementia-associated agitation. Two anticonvulsant medications, carbamazepine and valproic acid, have been studied in this regard. Controlled studies have documented a significant decrease in agitation in patients receiving carbamazepine,9–10 and open-label studies have shown efficacy for valproic acid.11–12 The use of these anticonvulsants to treat agitation has been reviewed by Grossman.13
Gabapentin, an anticonvulsant that appears to have a unique mechanism of antiseizure activity, is another drug that has been considered for the treatment of agitation in dementia. In one case study, a 68-year-old woman with Alzheimer's dementia, who had episodes of agitation and violence toward nursing home staff members, showed behavioral improvement when gabapentin was added to haloperidol.14 A case series of geriatric patients treated with gabapentin included 2 patients with dementia and agitation who responded favorably.15 Gabapentin appears to alter the activity of several neurotransmitters likely to be relevant to behavior disturbance in Alzheimer's disease, including gamma-aminobutyric acid (GABA), serotonin, and glutamate.16 It does not decrease the release of acetylcholine,16 a neurotransmitter whose deficiency is linked with cognitive impairment in Alzheimer's disease. In the elderly patient with dementia, who is likely to be on multiple medications, gabapentin may be a safer medication than either carbamazepine or valproic acid in that it is not metabolized by the liver and thus does not affect the metabolism of other medications.17 To further examine the efficacy of this drug, we report 4 elderly outpatients with dementia treated with gabapentin.
METHODS
All psychiatric medications, with the exception of donepezil, were discontinued at least 7 days prior to the initial evaluation. Patients then received gabapentin 300 mg/day, increased to 900 mg/day over 3 days. The dose was further increased at regular intervals over approximately 5 weeks. In order to maximize the chance of obtaining therapeutic benefit, patients received up to 2,400 mg/day as tolerated. The titration was modified for patients experiencing adverse effects. After at least 2 weeks at the maximum tolerated dose, a full medication taper was attempted over 14 days as feasible. Final evaluations were completed 1 week after taper completion.
Patients received a screening evaluation at the beginning of the study and were reassessed weekly. Psychometric evaluations occurred within 10 hours after the first daily dose. Baseline and follow-up measures included the Mini-Mental State Examination (MMSE)18 and the Overt Agitation Severity Scale (OASS).19 The OASS measures specific observable forms of agitation such as screaming, threatening, banging, hitting, and wandering, and considers both the intensity and frequency of symptoms. The mean score for psychiatric inpatients with agitation in one study19 was shown to be about 50 (maximum score on the OASS is 150).
All patients enrolled in the study met criterion A for the DSM-IV diagnosis of dementia, which requires an impairment in memory plus one other area of cognitive impairment. Four patients completed the study, and the data are reported below. An additional patient was enrolled but was discontinued from the study because of family concerns that were judged to be unrelated to study medication.
CASE STUDIES
Case 1. A 73-year-old married man developed ataxia, hallucinations, memory loss, and hostile behavior about 1 year prior to participation in the study. According to his wife, the patient had a previous history of “mini-strokes.” On evaluation, he showed moderate agitation and voiced suspicion of his wife's infidelity. He had a 20-year history of alcohol abuse, but reportedly had not been drinking alcohol for 3 years prior to the study. He had no previous exposure to psychiatric medication. His baseline MMSE and OASS scores were 26 and 21, respectively. An MRI showed lacunar infarcts and patchy areas of hyperintensity in the periventricular white matter bilaterally. The patient was diagnosed with mixed dementia. He was started on 300 mg/day of gabapentin, which was titrated to 2,400 mg/day over 35 days. He complained of headaches and dizziness, which eventually remitted. He became calmer and more alert. The OASS score declined to 16, and the MMSE score remained at 26. When gabapentin was tapered to a daily dose of 1,800 mg, the patient's agitation increased and his OASS score increased to 22. Gabapentin was increased to 2,400 mg/day. The patient and his family were pleased with the effect of the medication and chose to continue at this dose following completion of the study.
Case 2. An 80-year-old married woman with a history of cognitive decline presented with difficulty performing simple tasks, episodes of nighttime agitation, and visual hallucinations. She had previously been treated with methylphenidate 5 mg/day for depression; this was discontinued prior to evaluation for study entrance. At the time of enrollment, she had been receiving donepezil 10 mg daily for Alzheimer's dementia for more than 6 months. On evaluation, the patient had impaired gait and severe memory impairment. Her MMSE score was 15 and her OASS score was 9. A CT scan revealed moderate cortical atrophy. The patient was started on 300 mg/day of gabapentin titrated to 900 mg over 3 days. Episodes of agitation decreased in frequency, and her OASS score fell to 6. However, she developed apparent side effects including sedation, fatigue, apathy, and decreased appetite, and her MMSE score dropped to 11. After 2 weeks on 900 mg/day, the dosage of gabapentin was reduced gradually to 100 mg tid. At this dose, the patient was brighter and more alert and regained her appetite. Her OASS score was 3 and her MMSE score was 17. Only one further episode of agitation was reported. After gabapentin was discontinued, the caregiver reported that the patient had continued improvement in behavior, and her OASS score fell to 0.
Case 3. An 80-year-old married woman with a history of memory loss and paranoia, diagnosed with Alzheimer's dementia, presented with episodes of agitation and depressed mood. She had been receiving donepezil 5 mg/day for 7 weeks. She accused family members of “freeloading.” A previous MRI had revealed mild atrophy and minimal bilateral periventricular white matter ischemic changes. The patient had no history of receiving psychiatric medications. Her baseline MMSE and OASS scores were 23 and 11, respectively. The patient was started on 300 mg/day of gabapentin, increased to a peak dose of 2,400 mg/day over 36 days. She developed daytime drowsiness and nighttime insomnia, but her agitation fully remitted. Her MMSE score was 21 and her OASS score was 0. When the medication was discontinued, depression, perseverative behaviors, and agitation returned. Her appetite and attention span decreased. Her final MMSE and OASS scores were 27 and 18, respectively. Gabapentin was restarted at 1,800 mg/day and the patient showed immediate improvement. Daytime sedation did not recur.
Case 4. An 87-year-old woman presented with memory impairment, depression, and agitation associated with probable Alzheimer's-type dementia. She had previous medication trials with valproic acid and paroxetine. During the week prior to enrollment she was tapered off of lithium 150 mg bid, trazodone 50 mg hs, and nortryptiline 50 mg hs. These medications had been ineffective. At the time of enrollment, she had been receiving donepezil 5 mg/day for more than 6 months. The patient was argumentative and combative toward her daughter and home attendants. A CT scan revealed moderate cerebral and cerebellar atrophy. Her baseline MMSE score was 24 and baseline OASS score was 36. The patient was started on 300 mg tid of gabapentin, increased to 2,400 mg/day over 33 days. Her MMSE and OASS scores at this dose were 26 and 17, respectively, indicating an improvement in agitation. The medication did not produce adverse effects. When the dose was lowered to 1,800 mg/day, the caregiver reported that the patient became more irritable, and her OASS score rose to 39. The gabapentin taper was halted. At a follow-up visit 6 months later, the patient was receiving 2,100 mg/day of gabapentin and had an OASS score of 11.
DISCUSSION
Figure 1 shows the OASS scores and dosage of gabapentin for each patient. Three patients were successfully titrated to the full peak dose of 2,400 mg/day. One patient (Case 2) experienced sedation and disorientation, with a reduction in her MMSE score from 15 to 11, while receiving 900 mg/day. Her dose was reduced to 300 mg/day. Other side effects of gabapentin that were observed in these patients included headaches, dizziness, and reduced ambulation. In most instances, side effects were either tolerable or transient and did not prevent full titration in 3 of 4 cases.
As shown in Figure 1, the mean OASS score declined almost 50%, from 19.25 to 9.75, during treatment with gabapentin. Clinical improvement occurred in the following symptoms: vocal perseverating, cursing, threatening, moaning, crying, task perseverating, and hitting. Three of 4 patients continued on gabapentin after the study was completed. In Case 2, agitation did not recur during the tapering phase, so there was no indication for further treatment with gabapentin. Using plus or minus 2 points as the threshold for clinically significant change, we found that MMSE scores were not affected by gabapentin.
Limitations of the study include its small sample size, the use of open-label treatment, and the heterogeneity of the sample with regard to the etiology of dementia and the presence of comorbid depression and psychosis. Although agitation tends to be more persistent than other behavioral symptoms of dementia,20 spontaneous improvement may occur—as evidenced in Case 2, where agitation continued to decline after medication withdrawal. Further, because of the use of high doses of gabapentin, some of the improvement in agitation may have resulted from the sedating effects of the medication. In spite of these shortcomings, this preliminary study, applying objective measures of agitation, suggests a potential role for gabapentin in the treatment of agitation associated with dementia. Further research in this area, with an emphasis on determining the optimal dose of gabapentin, is warranted.
ACKNOWLEDGMENTS
The authors thank Ms. Jean Zarate, B.S., for editorial and research assistance.
This study was funded by a research grant from Parke-Davis.
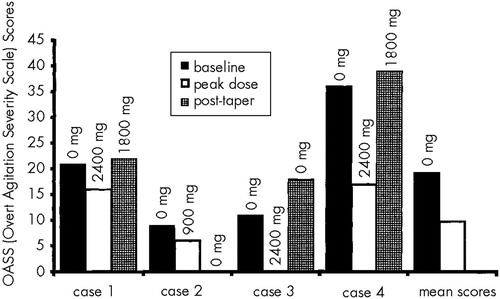
FIGURE 1. Overt Agitation Severity Scale scores of patients with dementia treated with gabapentin at baseline, peak dose, and after attempted medication taperActual dose of gabapentin at time of rating is indicated. The mean score for post-taper was omitted because not all patients could be discontinued from medication.
1 Rabins PV, Mace NL, Lucas MJ: The impact of dementia on the family. JAMA 1982; 248:333–335Crossref, Medline, Google Scholar
2 Deutsch LH, Rovner BW: Agitation and other noncognitive abnormalities in Alzheimer's disease. Psychiatr Clin North Am 1991; 14:341–351Crossref, Medline, Google Scholar
3 Cohen-Mansfield J, Billing N: Agitated behaviors in the elderly: a conceptual review. J Am Geriatr Soc 1986; 34:711–721Crossref, Medline, Google Scholar
4 Teri L, Lartson EB, Reifler BV: Behavioral disturbance in dementia of the Alzheimer's type. J Am Geriatr Soc 1988; 36:1–6Crossref, Medline, Google Scholar
5 Reisberg B, Borenstein J, Salob SP, et al: Behavioral symptoms in Alzheimer's disease: phenomenology and treatment. J Clin Psychiatry 1987; 48(suppl 5):9–15Google Scholar
6 Schneider LS, Pollock VE, Lyeness SA: A meta-analysis of controlled trials of neuroleptic. J Am Geriatr Soc 1990; 38:553–563Crossref, Medline, Google Scholar
7 Kunik ME, Yudofsky SC, Silver JM, et al: Pharmacologic approach to the management of agitation associated with dementia. J Clin Psychiatry 1994; 55(suppl 2):13–17Google Scholar
8 Herrmann N, Rivard M, Flynn M, et al: Risperidone for the treatment of behavioral disturbances in dementia: a case series. J Neuropsychiatry Clin Neurosci 1998; 10:220–223Link, Google Scholar
9 Tariot PN, Erb R, Leibovici A, et al: Carbamazepine treatment of agitation in nursing home patients with dementia: a preliminary study. J Am Geriatr Soc 1994; 42:1160–1166Google Scholar
10 Tariot PN, Erb R, Podgorski CA, et al: Efficacy and tolerability of carbamazepine for agitation and aggression in dementia. Am J Psychiatry 1998; 155:54–61Crossref, Medline, Google Scholar
11 Mellow AM, Solano-Lopez C, Davis S: Sodium valproate in the treatment of behavioral disturbances in dementia. J Geriatr Psychiatry Neurol 1993; 6:203–209Crossref, Google Scholar
12 Lott DA, McElroy SL, Keys MA: Valproate in the treatment of behavioral agitation in elderly patients with dementia. J Neuropsychiatry Clin Neurosci 1995; 7:314–319Link, Google Scholar
13 Grossman F: A review of anticonvulsants in treating agitated demented elderly patients. Pharmacotherapy 1998; 18:600–606Medline, Google Scholar
14 Regan WM, Gordon SM: Gabapentin for behavioral agitation in Alzheimer's disease. J Clin Psychopharmacol 1997; 17:59–60Crossref, Medline, Google Scholar
15 Sheldon L, Ancill RJ, Holliday SG: Gabapentin in geriatric psychiatry patients (letter). Can J Psychiatry 1998; 43:422–423Medline, Google Scholar
16 Taylor CP: Emerging perspectives on the mechanism of action of gabapentin. Neurology 1994; 44(suppl 5):S10–S16Google Scholar
17 Shorvan S, Stefan H: Overview of the safety of newer antiepileptic drugs. Epilepsia 1997; 38(suppl 1):S45–S51Google Scholar
18 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
19 Yudofsky SC, Kopecky HJ, Kunik M, et al: The Overt Agitation Severity Scale for the objective rating of agitation. J Neuropsychiatry Clin Neurosci 1997; 9:541–548Link, Google Scholar
20 Devanand DP, Jacobs DM, Tang MX, et al: The course of psychopathologic features in mild to moderate Alzheimer's disease. Arch Gen Psychiatry 1997; 54:257–263Crossref, Medline, Google Scholar



