Comparison of Clinical and Pathological Phenotypes in Two Ethnically and Geographically Unrelated Pedigrees Segregating an Equivalent Presenilin 1 Mutation
Abstract
At least 30 different missense mutations have been identified within the presenilin 1 (PS1) gene in pedigrees transmitting familial Alzheimer's disease. The authors investigated the clinical and pathological features of affected members of two pedigrees segregating a PS1 Met146Leu mutation. Genetic relationships between these pedigrees can be effectively excluded on the basis of genealogical data and the fact that although the amino acid substitution is identical, the nucleotide mutations are different. The clinical picture shows remarkable similarities in the neurological and the neuropathological findings between the two pedigrees. This general clinical and pathological concordance argues that much of the disease phenotype arises directly from the effects of the amino acid substitution within the PS1 protein itself. Clinical differences could arise from a direct effect of the difference in base sequence or, alternatively, from the effect of genetic or environmental modifiers.
At least 30 different missense mutations have been identified within the presenilin 1 (PS1) gene in pedigrees transmitting familial Alzheimer's disease (FAD). In the majority of instances the PS1 mutations have been associated with early-onset (before age 65), apparently autosomal dominant FAD. However, occasional instances of PS1 mutation in apparently sporadic Alzheimer's disease, or AD beginning after age 65, have been described.1 Moreover, distinct phenotypes associated with particular mutations have also been described.2
The majority of PS1 mutations have been described in Caucasian subjects. It is therefore unclear whether the general phenotypic uniformity, within a given genotype, of the clinical and neuropathological features arises because of the predominant effects of the PS1 mutations per se or because of a general similarity of the genetic background within which the mutations have been found. To address this question, we have investigated the clinical and pathological features of affected members of two pedigrees segregating a PS1 Met146Leu mutation. These pedigrees have different ethnic origins and reside in widely separated geographic locations. Our data reveal strong overall similarities in the clinical and pathological features that suggest the clinical phenotype is largely determined by the missense mutation itself. However, subtle differences in age at onset and in certain clinical features suggest that other genetic or environmental factors may concur to produce the phenotype.
METHODS
The pedigree diagrams and the clinical and neuropathological studies of the two related pedigrees of Calabrian origin (FAD4/N and Tor 1.1), along with the associated molecular genetic data, have been presented in depth elsewhere.3–5 These pedigrees now show a total of 111 persons known by personal examination, by medical record, or by history to have been affected with early-onset Alzheimer's disease, of whom 12 have been examined neuropathologically. Also, 14 obligate carriers have been identified, the earliest of whom lived at the end of the 17th century.
Preliminary clinical and pathological data on the South American Indian pedigrees (ARG1) resident in the northwestern region of Argentina have been presented elsewhere.6 Previously unpublished clinical and pathological data and previously unreported molecular genetic data on the ARG1 pedigree are described below.
Family Under Investigation
The ARG1 family pedigree is portrayed in Figure 1. It comprises more than 110 persons in 5 generations. We have clinical data to support the diagnosis of Alzheimer's disease in 11 patients, 4 of whom had pathological confirmation. Biological samples for genetic studies were obtained from the following affected persons:
CASE REPORTS
# III-9. The patient is now 47 years old. At age 45 he began to experience memory loss and behavioral disturbances characterized by apathy and depressive symptoms. On examination, the patient showed progressive memory impairment and defective problem-solving skills. He was irritable and presented with occasional aggressive outbursts. He denied the presence of symptoms and refused to collaborate with further neurological or neuropsychological evaluations.
# IV-30. The patient is now 41 years old. At age 34 he experienced progressive difficulties in his job as upholsterer, showing progressive behavioral problems and memory loss. The patient had a Mini-Mental State Examination score of 18 and a Global Dementia Scale rating of 5, and he performed 3 SD below the mean in all of the Alzheimer Disease Assessment Scale subtests.
# IV-31. The patient died in June 1996 after a 6-year course of dementing illness characterized by early behavioral disturbances, progressive cognitive impairment, extrapyramidal signs, myoclonus, and preterminal seizures. Brain samples were obtained at autopsy and are described in the Results section below.
Blood specimens were also obtained from the following unaffected subjects: III-6, III-7, IV-32, IV-33, IV-34, and the offspring of IV-30 and IV-31.
DNA Analysis
Genomic DNA and mRNA were prepared from buffy coat leukocytes as previously described.7 Sequencing of presenilin genes was done as described elsewhere.8 Briefly, PS1 reverse transcriptase-polymerase chain reaction (RT-PCR) products for the open reading frame were generated from the buffy coat leukocyte mRNA by using the PS1-specific oligonucleotide primers and RT-PCR conditions as previously described. The RT-PCR products were sequenced by using dye-labeled di-deoxynucleotide cycle sequencing on an ABI 372 automated DNA sequencer (Perkin Elmer Biosystems, Foster City, CA, USA). The resultant chromatograms were inspected manually and by the Sequence Navigator software package (Perkin Elmer). The Met146Leu mutation was confirmed to be present in genomic DNA, and its segregation within the ARG1 family was documented by using the genomic DNA-based assay as described previously.
RESULTS
DNA Analysis
Sequencing of the open reading frame PS1 from affected subjects revealed the presence of an A→T mutation at nucleotide 684, which is predicted to cause a Met→Leu missense substitution at codon 146. Although the nucleotide mutation is different, the resultant missense amino acid substitution is identical to that described for two large kindreds of Calabrian origin (FAD4/N and Tor 1.1) affected with early-onset FAD.
There is no known genealogical link between the South American and European pedigrees, and as far as can be discerned, there has been no intermarriage with Caucasians in the ARG1 pedigree. Furthermore, the mutation in PS1 for the ARG1 pedigree is ATG→TTG, while the mutation found in the FAD4/N and Tor 1.1 pedigrees is ATG→[011]CTG. As would be expected from the different DNA sequence mutations, there was also no sharing of alleles on the disease-bearing chromosome 14 between these families, confirming that there is no genetic relationship between them. This rules out the possibility of a common founder derived from the occupation of both Argentina and Calabria by the Spanish during the 17th century.
Clinical Comparison
Clinical descriptions of patients from the FAD4/N and Tor 1.1 pedigrees show that initial symptoms occur during the person's early forties and are usually characterized by behavioral disturbances, personality change, and forgetfulness.
The next stage is typified by progressive cognitive impairment, extrapyramidal signs, and the presence of psychotic symptoms. With further evolution of dementia, the patients rapidly become totally bedridden. Myoclonic jerks and epileptic seizures are the hallmarks of the terminal stage. Overall, the disease has an average duration of 7.32 years. We compared this clinical picture with that observed in patients from the ARG1 pedigree. The results are summarized in Table 1. We decided to set the significance level at P<0.005 in order to prevent alpha-inflation due to multiple between-group comparisons.
There is a significant difference in the frequency of cerebellar signs. Dynamic ataxia as evidenced by abnormal finger-nose and ankle-knee tests, nystagmus, truncal ataxia, and a broad-based cerebellar gait were present in 4 out of 11 ARG1 patients at some time during their clinical course. One of these patients also showed cerebellar hypoperfusion in a SPECT scan.6 In contrast, cerebellar signs were not observed in patients from the FAD4/N and Tor 1.1 pedigrees.
There is also a nonsignificant trend to a lower age at onset of FAD within the ARG1 pedigree (40 versus 42.7 years; P>0.01). The earlier age at onset in the ARG1 pedigree is more noticeable among ARG1 patients from the IV generation. The mean age at onset for the patients from the IV generation (n=4) is 35.0 years, whereas the mean age at onset for the patients from the third generation (n=7) is 42.14 years (Wilcoxon χ2=7.13, df =1,10, P<0.007). Further studies are required to discern whether this difference reflects ascertainment bias or a true biological anticipation effect.
Neuropathological Comparison
Neuropathological studies from the European FAD4/N and Tor 1.1 pedigrees have consistently shown the presence of widespread neuritic plaques and neurofibrillary degeneration in the cerebral cortex and granulovacuolar degeneration within the Ammon's horn. Amyloid angiopathy and spongiform changes have been occasionally observed.
We have recently studied brain samples from patient IV-31 of the ARG1 pedigree, who died in 1996. Pathological specimens were independently assessed in Argentina (E.C.) and Italy (J.F.).
CASE REPORT
A pathological diagnosis of Alzheimer's disease was established beyond doubt. Analysis of medial temporal structures in this brain disclosed abundant giant neuritic plaques, particularly in the amygdaloid nucleus. These were characterized by a fragmented amyloid center and by the presence of large dystrophic neurites. Microglial cells and diffuse astrocytic gliosis were associated with the plaques. Tangles and granulovacuolar degeneration were prominent in pyramidal cells. Isocortical regions of the temporal, frontal, and parietal lobes showed numerous diffuse plaques, especially in the second layer. Neuritic plaques were relatively infrequent. On the other hand, tangles were very numerous, with frequent ghost tangles, as would be expected from the evident neuronal loss. Astrocytic gliosis was widespread. There was also evidence of spongiform changes in middle and deep isocortical layers, particularly in the frontal lobe. No cortical Lewy bodies were detected. The white matter volume appeared to be reduced.
Pigmented neurons were not markedly rarefied, but neuronal loss was evidenced by the presence of free pigment and pigment-containing macrophages. A few Lewy bodies were found in pigmented neurons, but more frequently the latter harbored tangles, which were also found as ghost tangles tightly associated with pigment.
The primary neuropathological lesions that define Alzheimer's disease were certainly the same as those found in FAD4/N and Tor 1.1 index cases. In addition, microspongiosis has been described also in those cases as well as in several other patients with PS1 mutations.
A striking feature of the present case, however, is the occurrence of Lewy bodies in pigmented cells of the substantia nigra, along with an absence of senile plaques in this region. The opposite was found in patients from the FAD4/N kindred. The extreme abundance of giant neuritic plaques in the amygdaloid nucleus is also a particular feature of this case.
DISCUSSION
We have reported here the clinical and neuropathological features of two pedigrees segregating genomic DNA mutations that give rise to the PS1 Met146Leu missense substitution. Genetic relationships between these pedigrees can be effectively excluded on two grounds. First, from genealogical data it is unlikely that the ARG1 South American Indian pedigree has any relationship to the FAD4/N and Tor 1.1 pedigrees of Calabrian origin. Second, although the amino acid substitution is identical, the nucleotide mutations are different.
Despite the clear differences in the genetic origins of these two pedigrees, the clinical picture shows remarkable similarities in terms of the behavioral and cognitive disturbances as well as in the appearance of several additional neurological features including extrapyramidal signs, myoclonus, and seizures. There is also remarkable similarity in the neuropathological findings in the two pedigrees. Both pedigrees showed extensive neurodegeneration with abundant amyloid plaques and neurofibrillary tangles as well as the presence of microspongiosis.
This general concordance of the clinical and pathological features between the two pedigrees argues that much of the disease phenotype arises directly from the effects of the amino acid substitution within the PS1 protein itself. However, there were also subtle differences in the clinical phenotype between these pedigrees. Thus, there was a borderline significant difference in the age at onset and in the frequency of seizures, and there was a highly significant difference in the frequency of cerebellar signs. In addition, there were subtle pathological differences including the presence of Lewy bodies in pigmented cells of the substantia nigra, which occurs in the absence of senile plaques in the ARG1 pedigree but not in the Calabrian pedigrees. Because the clinical features that differentiate these two pedigrees were observed in several affected individuals, the notion that these differences arise from stochastic or random effects can be rejected.
These clinical and pathological differences could arise from a direct effect of the difference in base sequence (independent of the resulting amino-acid sequence), as hypothesized by Tanzi and Hyman.9 This effect could possibly occur in relation with allosteric effects of DNA conformation on transcriptional regulators10—a hint for a role of transcriptional factors in the expression of Alzheimer's disease. The clinical and pathological differences could be due, alternatively, to the effect of genetic modifiers or differences in environmental modifiers. The latter might be related either to the different geographic origins or the different cultural habits of these families. We should recall, however, that a previous quantitative study of phenotypes within family N/FAD411 could not evidence any influence of either genetic modifiers or environmental factors, although the latter were widely varied owing to the geographical dispersion of the kindred both in Europe and America. Although the difference in age at onset in the pedigrees under consideration is relatively small (approximately 3 years) and of borderline statistical significance, similar but more profound variations in age at onset have been observed in pedigrees segregating presenilin 2 (PS2) mutations. Given the overall similarities of the disease arising from PS1 and PS2 mutations, it will be of interest to learn whether the modifying factors are the same in PS1 and PS2 cases and whether they can be exploited therapeutically.
ACKNOWLEDGMENTS
The authors thank Dr. A. Leotta (Lamezia Terme), in whose laboratory histopathological specimens of ARG1 family have been studied. This work was supported through grants from the Medical Research Council of Canada, The Canadian Genetic Diseases Network, The Alzheimer Association of Ontario, The Howard Hughes Medical Research Foundation, the EJLB Foundation, the Telethon E0352 (A.C.B.), and the Associazione per la Ricerca Neurogenetica, Italy (A.C.B.). E.A.R. is a recipient of the Peterborough Burgess Fellowship. R.J. is supported by a grant from Eli Lilly & Co. to Asociación Lucha Mal de Alzheimer.
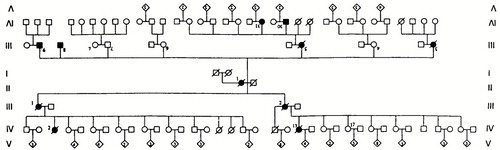
FIGURE 1. The ARG1 family pedigree
 |
1 Hardy J: Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci 1997; 20:154–159Crossref, Medline, Google Scholar
2 El Hachimi KH, Cervenakova L, Brown P, et al: Mixed features of Alzheimer disease and Creutzfeldt-Jakob disease in a family with a presenilin 1 mutation in chromosome 14. Amyloid: Int J Exp Clin Invest 1996, 3:223–233Google Scholar
3 Foncin JF, Salmon D, Supino-Viterbo V, et al: Démence presenile d'Alzheimer transmise dans une famille etendue [Alzheimer's presenile dementia transmitted in an extended family]. Rev Neurol (Paris) 1985; 141:1904–2102Google Scholar
4 Bruni AC, Montesi MP, Gei G, et al: The common origin of familial Alzheimer's disease in Calabria, in Alzheimer's Disease: Basic Mechanisms, Diagnosis and Therapeutic Strategies, edited by Iqbal K, McLachlan DRC, Winblad B, et al. New York, Wiley, 1991, pp 451–455Google Scholar
5 Sherrington R, Rogaev EI, Liang Y, et al: Cloning of a gene bearing missense mutations in early onset familial Alzheimer's disease. Nature 1995; 375:754–760Crossref, Medline, Google Scholar
6 Mangone C, Castano E, Levy E, et al: Early onset Alzheimer's disease in a South American pedigree from Argentina. Acta Neurol Scand 1995; 91:6–13Crossref, Medline, Google Scholar
7 St. George-Hyslop PH, Haines JL, Rogaev E, et al: Genetic evidence for a novel familial Alzheimer's disease locus on chromosome 14. Nat Genet 1992; 2:330–334Crossref, Medline, Google Scholar
8 Liu Q, Sommer SS: Restriction endonuclease fingerprinting (REF): a sensitive method for screening mutations in long contiguous segments of DNA. Biotechniques 1995; 18:470–476Medline, Google Scholar
9 Tanzi R, Hyman B: Alzheimer's mutation. Nature 1991; 350:564Crossref, Medline, Google Scholar
10 Lefstin JA, Yamamoto KR: Allosteric effect of DNA on transcriptional regulators. Nature 1998; 392:885–888Crossref, Medline, Google Scholar
11 Bruni AC, Montesi MP, Salmon D, et al: Alzheimer's disease: a model from the quantitative study of a large kindred. J Geriatr Psychiatry Neurol 1992; 5:126–131Crossref, Medline, Google Scholar



