SPECT Findings in Mentally Retarded Autistic Individuals
Abstract
The authors examined specific deficits of cerebral blood perfusion in autistic patients as measured with [99mTc]HMPAO single-photon emission computed tomography (SPECT). The study, conducted in an outpatient clinic setting, included a consecutive series of 30 patients with autism and 14 patients with mental retardation but no autism comparable in chronological age, mental age, height, weight, and head circumference. All participants were examined with a comprehensive psychiatric and neuropsychological battery and received a [99mTc]HMPAO SPECT scan. Autistic patients had significantly lower perfusion than the control group in the following brain regions: right temporal lobe (basal and inferior areas), occipital lobes, thalami, and left basal ganglia. The study demonstrated significant perfusion deficits in specific brain areas of moderately to severely mentally retarded autistic patients.
Autism is a behavioral syndrome defined by the presence of pervasive social deficits, communication abnormalities, and restricted, repetitive, and stereotyped patterns of behaviors.1 Although autism is etiologically heterogeneous and genetic factors play a primary role,2 the biological mechanisms of this disorder remain unknown.
In one of the initial positron emission tomography (PET) studies in autistic individuals, Rumsey et al.3 reported more extreme relative metabolic rates and asymmetries in autistic individuals compared with a group of normal control subjects. A reanalysis of this data4 demonstrated reduced correlations of metabolic activity between different brain regions (mainly subcortical structures and frontal-parietal areas) in autistic individuals compared with normal control subjects. The authors suggested this abnormal correlation pattern may produce an imbalance in inhibitory circuits related to attention and inward/outward-directed behaviors.
Single-photon emission tomography (SPECT) had a late development compared with PET, having a lower tomographic resolution and lacking the capacity of PET for direct metabolic measurements. However, in most psychiatric disorders, brain perfusion (as assessed with SPECT) and brain metabolism (as assessed with PET) are tightly coupled.5
Zilbovicius et al.6 used xenon SPECT to assess 21 autistic children and 14 age-matched nonautistic control subjects with developmental language disorder; they did not find significant between-group differences in global or regional cerebral perfusion. Using the same methodology, Chiron et al.7 examined 18 autistic patients and 10 normal control subjects and found a significantly lower cerebral blood flow in the posterior-inferior prefrontal and posterior temporal lobe regions of both hemispheres in autistic patients compared with control subjects. In a qualitative study, Gillberg et al.8 found left temporal perfusion deficits in 31 autistic patients, and a similar finding was recently reported by Mountz et al.9 There are several limitations to these studies, such as small sample size and control subjects not always comparable in mental age. Notably, all of these studies have been of high-functioning autistic individuals. Autism is associated with mental retardation in approximately 70% of cases, and this is very likely an index of etiologic heterogeneity in this disorder. Thus, findings from studies in high-IQ autistic individuals cannot necessarily be generalized to the population of autistic individuals with mental retardation. Given the large proportion of autistic individuals with mental retardation and the possibility that underlying mechanisms may differ in autistic individuals with and without mental retardation, in the present study we examined a series of 30 mentally retarded autistic subjects (most with moderate or severe mental retardation) and a group of 14 nonautistic individuals with comparable mental age, using [99mTc]HMPAO SPECT.
METHODS
Subjects
A series of 30 subjects who attended the Autism Clinic at the Raúl Carrea Institute of Neurological Research were included in the present study. All subjects had an MRI scan as part of their initial diagnostic evaluation. Subjects with a positive history of medical or neurological disorders (except for seizures in 2 patients) were excluded. A pediatric neurologist carried out a structured neurological evaluation to rule out neurological deficits, dysmorphic features, or neurocutaneous abnormalities. On the basis of this evaluation, 1 patient with tuberous sclerosis and 1 patient with Sturge-Weber disease were excluded from the study. All parents of autistic subjects were interviewed with the Autism Diagnostic Interview (ADI),10 and all subjects met both the DSM-IV11 and the ADI algorithm10 criteria for autistic disorder. Only autistic individuals with an IQ lower than 70 were included in the study.
Control subjects were 14 patients with mental retardation who attended the Learning Disorders Clinic at our institute. None of the control subjects had a history of medical or neurological disorder (except for seizures in 2 patients). Control subjects who had mental ages within the range of the autistic group were selected from a larger pool of 35 individuals with mental retardation who received an MRI scan as part of an ongoing imaging study. All control subjects were assessed with the ADI, and none met the ADI algorithm criteria for either autism or “pervasive developmental disorder not otherwise specified.”
Psychiatric Examination
The following psychiatric examinations were administered:
Autism Diagnostic Interview (ADI):10 The ADI is a semistructured diagnostic interview for autism. The ADI has been shown to be reliable, and it adequately discriminates autistic individuals from mental age–matched nonautistic comparison groups.
Childhood Autism Rating Scale (CARS):12 The CARS is a 15-item behavioral rating scale developed to identify children with autism and to distinguish them from developmentally handicapped children without autism syndrome. The CARS has been demonstrated to have high reliability and to be a valid measure of the diagnosis of autism.
Aberrant Behavior Checklist (ABC):13 The ABC is a 58-item rating scale that measures pathological behaviors in moderately to profoundly mentally retarded individuals with developmental disorders. It is divided into five dimensions: irritability, lethargy, stereotypic behaviors, hyperactivity, and excessive speech.
Neuropsychological Examination
All 30 autistic and 10 of the 14 control subjects were assessed with the Leiter International Performance Scale,14 a measure of nonverbal IQ. Because of the severity of mental retardation in most of our sample, the Wechsler Intelligence Scale for Children (WISC)15 was administered successfully in only 4 control subjects and in none of the autistic patients. The Performance IQ of the WISC was used as an estimate of nonverbal IQ in these subjects.
SPECT Examination
This study was approved by the Ethics Review Committee of the Raúl Carrea Institute of Neurological Research. Written consent was obtained from caregivers and, where possible, subjects (5 autistic individuals and 9 control subjects) after a full explanation of the study. A SPECT study was carried out within 1 week after the psychiatric examination. Technetium-99m d,l,hexamethylpropyleneamine oxime ([99mTc]HMPAO; Ceretec [exametazime], Amersham International) 25 mCi was injected intravenously into an antecubital vein. Subjects sat with eyes closed and ears unplugged in a quiet room with dim lights. Fifteen minutes after the injection, subjects were placed in a supine position with the orbitomeatal line positioned vertically, centered in the field of view. Five autistic patients and 3 control subjects were anesthesized before the MRI and SPECT scanning, using inhalatory anesthesia with either halothane or sevoflurane ([99mTc]HMPAO was always injected 1 hour before the anesthesia). The alignment was carried out with vertical and horizontal laser beams, and the subject's head was physically restrained in place. SPECT was carried out on a General Electric 400 AC/T rotating gamma camera attached to a Starcam 3200 computer. The resolution of the system has been measured to be 14 mm full width at half maximum in the plane of reconstructed transverse sections. An MRI scan was carried out in every subject, and slices 5 mm thick were obtained in the axial, coronal, and sagittal planes by use of a fast sequence. To minimize motion during scanning, caregivers were always allowed to stay next to the scanner.
SPECT studies were carried out with a high-resolution collimator and a 64×64 matrix. There were 64 images obtained over 360 degrees, with a 30-second acquisition time and a zoom of 1.6. Processing was carried out with Butterworth filtering, a critical frequency of 0.44, and a slice width of 1 pixel. Reconstructed brain slices were then reoriented in the orbitomeatal line by using the sagittal and axial views, and a set of 30 axial, sagittal, and coronal sections at 6.4-mm increments was obtained. This procedure was carried out by using Starcam software. Square regions of interest (ROIs) consisting of 3×3 pixels (voxel [3×3×1 pixels]=2.35 cm3) were used to obtain activity ratios in axial slices, taking the cerebellum as a reference. Specific ROIs were identified by using the atlas of Matsui and Hirano16 and defined for each MRI scan. Measurements (anterior, middle, and posterior) were carried out for each of the following brain areas: frontal orbital, frontal superior (dorsal), temporal basal, temporal inferior, temporal superior, parietal, occipital lobes, thalamus, and basal ganglia. These measurements were averaged for each region in the right and left hemispheres. ROIs were also placed in the cerebellum. To determine the activity ratio (brain region/cerebellum), the counts per ROI of each cortical area were divided by the average counts per ROI found in each cerebellar hemisphere in the region with the highest average count. This ratio was used as a measure of regional cerebral blood flow because none of the previous SPECT studies in autism showed perfusion abnormalities in the cerebellum. All SPECT measurements were carried out by a neuroradiologist blinded to the clinical data. The intrarater and interrater reliabilities of these measurements have been reported previously.17
Statistical Analysis
Statistical analysis was carried out with analysis of covariance (ANCOVA) and post hoc Tukey tests. Frequency distributions were calculated by using chi-square tests and a Yates' correction for expected cell sizes smaller than 5. All probability values are two-tailed.
RESULTS
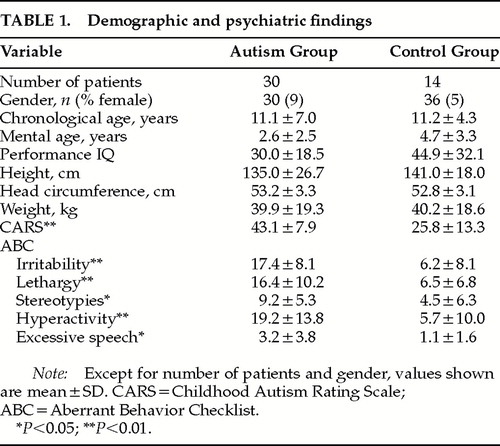
Demographic and Psychiatric Findings (Table 1)
There were no significant differences in gender, chronological age (t=0.32, P=0.74), mental age (t=0.66, P=0.50), or performance IQ (t=1.59, P=0.12) between autistic individuals and control subjects. Height, head circumference, and weight were also comparable. As expected, autistic individuals had significantly higher CARS scores than nonautistic control subjects (t=5.38, df=42, P<0.0001). Autistic individuals also showed significantly higher scores than the control group on all five ABC domains: irritability (t=6.21, df=42, P<0.0001), lethargy (t=3.27, df=42, P<0.01), hyperactivity (t=5.71, df=42, P<0.01), excessive speech (which also assesses repetitive language and stereotypic speech; t=1.95, df=42, P=0.05), and stereotypies (t=2.51, df=42, P<0.05).
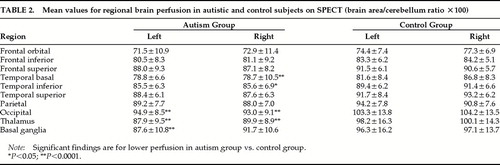
MRI and SPECT Findings (Table 2)
All MRI scans were read as normal by a board-certified neuroradiologist. In a recent study that included most of the present sample, we demonstrated the autistic group to have significantly smaller corpus callosum measurements compared with the control group,18 but no significant between-group differences were found in cerebellar vermis measurements.
A three-way ANCOVA with repeated measures for SPECT measurements (group×region×side; covariate: mental age) showed a significant group effect (F=8.13, df=1,41, P<0.01, autistic individuals showing significantly lower overall brain perfusion than the control group); a significant group×region×side interaction (F=2.02, df=9,378, P<0.05); and a trend for a significant group×region interaction (F=1.81, df=9,378, P=0.06). Post hoc analyses demonstrated that autistic patients had significantly lower perfusion than the control group in the following brain areas: right basal temporal (P<0.0001), right inferior temporal (P<0.05), right and left occipital (P<0.0001), right and left thalamus (P<0.0001), and left basal ganglia (P<0.0001). When those individuals who underwent anesthesia or had a history of seizures were excluded from the statistical analysis, there still was a significant group×region interaction (F=1.96, df=9,270, P<0.05).
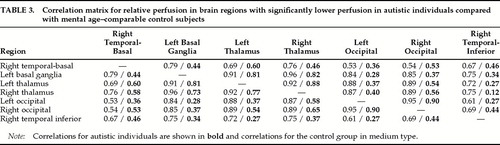
To assess the evidence for abnormal connectivity between the above brain areas, we calculated a correlation matrix between these areas for both autistic individuals and control subjects (Table 3). Using a conservative alpha=0.001 (equivalent to an r of 0.75 for the control group), the control group had 26 of 42 correlations (62%) above this r-value, as compared to only 8 of 42 correlations (19%) for the autistic group (χ2=18.4, df=1, P<0.0001).
To explore clinical correlates of the SPECT results, we calculated correlations between the three ADI domains and the relative perfusion in brain areas that differed significantly between autistic and control subjects. We found significant correlations (P<0.01) between scores for ritualistic-repetitive behaviors and both left and right relative thalamic perfusion (r=–0.39 and r=–0.42, respectively). None of the remaining correlations were significant at the P<0.01 level. Within the ritualistic-repetitive behavior domain, relative thalamic perfusion was significantly correlated with the following items: resistance to change in the environment (right thalamus, r=–0.44, P<0.01); unusual attachments (right and left thalamus, r=–0.40, P<0.01, and r=–0.37, P<0.05, respectively); difficulties with minor changes in routine (right and left thalamus, r=–0.37, P<0.05, and r=–0.35, P<0.05, respectively); and unusual sensory interests (right thalamus, r=–0.40, P<0.01).
DISCUSSION
We carried out [99mTc]HMPAO SPECT studies in a series of autistic individuals and a group of nonautistic control subjects with mental retardation and mental age comparable to those of the autistic group. Autistic subjects showed significantly lower perfusion in the right temporal and both occipital lobes, left and right thalami, and left basal ganglia.
Several limitations of our study should be pointed out. Autistic individuals and several control subjects were assessed with different neuropsychological instruments. However, these assessments are well standardized in autistic samples and provide a valid estimate of mental age. Another limitation is that some of the autistic subjects and control subjects had to be anesthesized before the SPECT and MRI studies. However, anesthesia was always administered no less than one hour after the injection of [99mTc]HMPAO (i.e., when the radiotracer uptake is in the steady state). Ideally, our study would have also included a normal control group, but there are serious objections to exposing normal children to radioactive substances. Our study did not include high-functioning autistic individuals, and whether there are significant differences in regional brain perfusion between high- and low-functioning autistic subjects will have to be examined in future studies.
Our study included primarily autistic patients with moderate to severe mental retardation and a control group comparable in chronological and mental age, Performance IQ, and morphometric variables such as height, weight, and head circumference. Autistic individuals had significant perfusion deficits in ventral brain areas such as the basal and inferior temporal-occipital regions, and in subcortical structures such as the thalami and left basal ganglia. The question that now arises is what relevance these findings have to the brain mechanism underlying autism.
Recent neuroimaging studies demonstrate brain abnormalities in autistic individuals, such as a smaller size of the corpus callosum;18,19 enlargement of the cerebellum and the parietal, temporal, and occipital lobes;20 and an increase in basal ganglia volume in high-functioning autistic subjects.21 Postmortem studies also showed developmental abnormalities in temporal lobe structures and cerebellum.22 Our finding of significantly lower temporal and occipital perfusion in autistic subjects suggests that the structural abnormalities of these brain areas may result in reduced cortical activity.
Sears et al.21 reported significant correlations between lower caudate volumes, as measured with MRI, and higher scores in the ritualistic-repetitive domain of the ADI. In the present study, we found a significant negative correlation between left and right thalamic perfusion and ritualistic-repetitive behaviors, suggesting that subcortical dysfunction may be related to the production of sterotyped and ritualistic behavior in low-functioning autistic individuals.
We calculated correlation matrices for relative perfusion in the brain areas found to be significantly different between autistic and control subjects. We found these correlations to be more robust in the control group than in the autistic group, supporting Rumsey and co-workers'3,4 finding of reduced correlations of metabolic activity between different brain regions in autistic individuals compared with normal control subjects. It is important to note, however, that our results were generated after a post hoc analysis that included a large set of correlations, and they should be considered in the light of this methodological limitation.
It is still unclear how the above brain abnormalities may account for the social and communication deficits and stereotyped-repetitive behaviors that define autism. Our present SPECT findings may help to explain several behavioral features of autism, such as impulsive and aggressive behaviors (to self and others), motor disinhibition (such as stereotypic and manneristic movements and echophenomena), and deficits in planning, sequencing, and attention. In recent years, Starkstein and Robinson23 examined the neuroanatomical correlates of disinhibition syndromes (e.g., euphoria, hyperactivity, irritability, aggressive outbursts, and impulsive behaviors) in adult individuals with focal brain lesions. They found significant involvement of paleocortical brain areas such as the inferior temporal and orbitofrontal brain regions. Pandya and Yeterian24 recently suggested that ventral temporal and middle prefrontal areas as well as paleocortically derived regions of the thalamus and basal ganglia may provide response modulation, planning and sequencing, means of holding events “online,” and attentional resources. Thus, dysfunction of these brain areas, as demonstrated in the present study, could produce some of the emotional and behavioral disorders usually described in autistic subjects.
ACKNOWLEDGMENTS
This study was partially supported by grants from the Raúl Carrea Institute of Neurological Research-FLENI, the Fundación Perez Companc, and National Institute of Mental Health Grant K02-MH01568 to J.P.
 |
 |
 |
1 Goodman R: Infantile autism: a syndrome of multiple primary deficits. J Autism Dev Disord 1989; 19:409–424Crossref, Medline, Google Scholar
2 Bailey A, Philips W, Rutter M: Autism: towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J Child Psychol Psychiatry 1996, 35:877–900Google Scholar
3 Rumsey J, Duara R, Grady C, et al: Brain metabolism in autism. Arch Gen Psychiatry 1985; 42:448–455Crossref, Medline, Google Scholar
4 Horwitz B, Rumsey J, Grady C, et al: Cerebral metabolic landscape in autism: intercorrelations of regional glucose utilization. Arch Neurol 1988; 45:749–755Crossref, Medline, Google Scholar
5 Trivedi MH, Husain M, Devous M: Functional brain imaging: SPECT—basic and technical considerations, in Brain Imaging in Clinical Psychiatry, edited by Krishnan KRR, Doraiswamy PM. New York, Marcel Dekker, 1997, pp 47–62Google Scholar
6 Zilbovicius M, Garreau B, Tsourio N, et al: Regional cerebral blood flow in childhood autism: a SPECT study. Am J Psychiatry 1992; 149:924–930Crossref, Medline, Google Scholar
7 Chiron C, Bulteau C, Leon F: SPECT of the brain in childhood autism: evidence for a lack of normal hemispheric asymmetry. Dev Med Child Neurol 1995; 37:849–860Crossref, Medline, Google Scholar
8 Gillberg C, Bjure J, Uverant P, et al: SPECT (single photon emission computed tomography) in 31 children and adolescents with autism and autistic-like conditions. Eur Child Adolesc Psychiatry 1993; 2:50–59Crossref, Medline, Google Scholar
9 Mountz JM, Tolbert LC, Lill DW, et al: Functional deficits in autistic disorder: characterization by technetium-99m-HMPAO and SPECT. Nucl Med 1995; 36:1156–1162Google Scholar
10 LeCouteur A, Rutter M, Lord C, et al: Autism Diagnostic Interview: a standardized investigator-based instrument. J Autism Dev Disord 1989; 19:363–387Crossref, Medline, Google Scholar
11 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994Google Scholar
12 Schopler E, Reichler RJ, Renner BR: The Childhood Autism Rating Scale (CARS) for diagnostic screening and classification of autism. New York, Irvington, 1986Google Scholar
13 Krug DA, Arick J, Almond P: Behavior checklist for identifying severely handicapped individuals with high levels of autistic behavior. J Child Psychol Psychiatry 1980; 21:221–229Crossref, Medline, Google Scholar
14 Arthur G: The Arthur adaptation of the Leiter International Performance Scale. Chicago, Psychological Services Press, 1952Google Scholar
15 Wechsler D: Wechsler Intelligence Scale for Children–III. New York, Psychological Corporation, 1991Google Scholar
16 Matsui T, Hirano A: An Atlas for the Human Brain for Computerized Tomography. New York, Igaku-Shoin, 1978Google Scholar
17 Starkstein SE, Vazquez S, Petracca G, et al: A SPECT study of delusions in Alzheimer's disease. Neurology 1994; 44:1055–2059Google Scholar
18 Manes F, Piven J, Vrancic D, et al: An MRI study of the corpus callosum and cerebellum in mentally retarded autistic individuals. J Neuropsychiatry Clin Neurosci 1999; 11:470–474Link, Google Scholar
19 Piven J, Bailey J, Ranson BJ, et al: An MRI study of the corpus callosum in autism. Am J Psychiatry 1992; 154:1051–1055Google Scholar
20 Piven J, Arndt S, Bailey J, et al: Regional brain enlargement in autism: a magnetic imaging study. J Am Acad Child Adolesc Psychiatry 1996; 35:530–536Crossref, Medline, Google Scholar
21 Sears LL, Vest C, Mohamed S, et al: An MRI study of the basal ganglia in autism. Prog Neuropsychopharmacol Biol Psychiatry 1999; 23:613–624Crossref, Medline, Google Scholar
22 Bauman ML, Kemper TL: Neuroanatomic observations of the brain in autism, in The Neurobiology of Autism, edited by Bauman ML, Kemper TL. Baltimore, Johns Hopkins University Press, 1994, pp 119–145Google Scholar
23 Starkstein SE, Robinson R: Mechanism of disinhibition after brain lesions. J Nerv Ment Dis 1997; 185:108–114Crossref, Medline, Google Scholar
24 Pandya DN, Yeterian EH: Morphological correlations of human and monkey frontal lobe, in Neurobiology of Decision-Making, edited by Damasio AR, Damasio H, Christen R. Berlin, Springer, 1996, pp 14–46Google Scholar



