Relationships Among Apathy, Depression, and Cognitive Impairment in HIV/AIDS
Abstract
This study was designed to determine whether apathy is associated with neurocognitive symptoms and/or depressive symptoms in HIV/AIDS and also whether apathy is associated with patient expectancies about antiretroviral medication adherence. Seventy-five HIV+ homosexual men and 58 HIV+ women were assessed for depressive disorders and symptoms. Neuropsychological tests measured attention, concentration, learning, memory, executive function, and psychomotor speed. Other measures included Marin's Apathy Evaluation Scale, the Adherence Determinants Questionnaire, CD4 cell count, and HIV RNA viral load. Apathy was consistently related to depression and unrelated to neuropsychological impairment. Patient expectancies regarding medication adherence were unrelated to apathy when the analysis was controlled for depressive symptoms.
The relatively recent interest in apathy in the psychiatric literature—spurred by development of a validated assessment method—coincides with growing interest in the concept among clinicians treating patients with HIV/AIDS. Its salience for these providers is related to its possible role in understanding behavior such as protracted failure to seek medical care after being informed of HIV-seropositive (HIV+) status or nonad-herence to prescribed medication regimens among patients who do seek medical treatment. Treatment delay and medication nonadherence can permit unnecessary disease progression in this era of effective antiretroviral combination therapy.
Apathy refers to a cluster of symptoms reflecting lack of motivation manifested in motoric, emotional, and cognitive domains. The construct, according to Marin,1 entails “simultaneous diminution in the overt behavioral, cognitive and emotional concomitants of goal-directed behavior”; these are conceptualized as components, not subtypes. Motoric apathy is characterized by the tendency not to initiate a new motor activity unless externally prompted. Cognitive apathy is defined as indifference, a generalized loss of interest, decrease in goal-directed thought content (e.g., “I have no plans”), diminished motivation associated with executive functions, and sometimes decreased verbal fluency. Emotional apathy is defined as diminished intensity or persistence of emotion, or placidity, relative to the importance of some goal-directed thought or event.
In the psychiatric literature, Marin and colleagues1–5 have been prime contributors to both the delineation and the assessment of the behavioral condition of apathy. They consider it to be often related to, but discriminable from, depression. In addition to the apathy seen in patients with major depression, apathy may also be observable in patients who do not manifest other affective symptoms (e.g., guilt) or somatic symptoms such as sleep and appetite disturbances. Marin's group3 studied 85 patients with stroke or Alzheimer's disease as well as 31 “normal” elderly subjects, using his Apathy Evaluation Scale and the 17-item Hamilton Rating Scale for Depression. Levy et al.6 studied 154 patients with various dementing disorders, using the Apathy subscale of the Neuropsychiatric Inventory. Neither study found a statistically significant relationship between apathy and depressive symptoms in demented or normal subjects.
In the general medical literature, apathy is associated with a wide range of psychiatric and neurologic conditions, ranging from depression and the negative symptoms of schizophrenia to the neuropsychiatric manifestations of neurologic diseases such as Huntington's, Parkinson's, and Alzheimer's diseases. Among HIV+ subjects, it has not been established whether apathy is a symptom of a purely psychiatric disorder (e.g., depression) or a behavioral manifestation of a primary neurologic condition, or both. Although rates of current major depression are not substantially elevated in non–drug-using HIV+ men and women, and its initial onset often antedates HIV infection,7 depression is nevertheless the most commonly observed Axis I psychiatric disorder in this population. Similarly, mild to moderate cognitive impairment has been reported in about 40% of patients with symptomatic HIV illness, increasing to more than 50% for patients with AIDS-defining conditions.8,9 Available evidence indicates that depression is not a necessary concomitant of cognitive impairment, although given their distributions they sometimes co-occur without a causal association.10–12 In HIV-associated dementia, apathy rather than depression is considered the predominant affect.13
Castellon et al.14 investigated the relationship between apathy, depression, and cognitive performance in HIV infection. Their sample included 26 subjects with AIDS-defining conditions, 22 subjects with absent to moderate HIV symptoms, and 21 HIV-seronegative control subjects. Apathy was measured by a subscale of the clinician-rated Neuropsychiatric Inventory. Depression was assessed with the Beck Depression Inventory (BDI), and neuropsychological performance was assessed with measures of reaction time and working memory. Scores on the apathy and depression measures were significantly correlated (r=+0.63, P<0.001), with apathy more strongly related to the cognitive than to the somatic depressive subscales of the BDI. However, apathy, but not depression, was found to be associated with working memory deficits among HIV+ subjects. Higher apathy scores and poorer working memory characterized the subjects with AIDS in contrast to the other two groups, although apathy was not correlated with CD4 cell count. Castellon et al. concluded that apathy, independent of depression, may indicate central nervous system (CNS) involvement in HIV infection.
This study was designed to investigate whether apathy in HIV is a result of psychological factors or if it is related to the underlying pathophysiology of HIV with respect to brain systems. Two major goals were formulated. The first was to extend the work of Castellon and colleagues, using a larger sample of HIV+ subjects with more advanced disease, a more specific measure of apathy, and broader battery of neuropsychological tests, to further explore the interrelatedness of apathy, depression, and cognitive deficits in HIV+ patients. In this context, we sought to study the relationship, if any, of apathy to HIV illness stage, using laboratory markers of illness progression and antiretroviral medication regimen. The second major goal was to investigate the relationship between apathy and patients' attitudes about medication adherence.
METHODS
Sample
Two groups of HIV+ adults were included. The first consisted of 75 HIV+ homosexual men who were enrolled in a larger cohort study with semiannual assessments conducted at our clinic. Half had no evidence of cognitive impairment in the prior 18 months covering three assessment visits. The others had been classified as having neuropsychological impairment (scores at least 1.5 standard deviations below the group means on two or more neuropsychological tests) at least twice in the preceding three assessments. In addition, 58 women were newly recruited, unselected for cognitive status. Inclusion criteria for both samples were the presence of HIV infection, sobriety for the past 12 months, history of at least 1 year of paid employment, and sufficiently stable health to participate in a 3-hour evaluation. Potential neurocognitive confounders were not exclusion criteria, but were assessed. The men were recruited for their initial participation in 1995, the women in 1998 through the same methods: flyers at HIV community organizations, notices in HIV-related newsletters, and word of mouth.
Measures
Note: Except where indicated, numerically higher scores signify a higher level of the condition being measured.
Psychiatric/Psychosocial: Mood modules, the psychotic and substance abuse screens (and the substance abuse modules as indicated) of the Structured Clinical Interview for DSM-IV (SCID)15 were administered to provide current (past month) and lifetime diagnoses. The structured version of the 17-item Hamilton Rating Scale for Depression (Ham-D),16 a clinician-administered assessment, and the BDI,17 a 21-item self-report scale, were administered to assess severity of depressive symptoms. Following Marin et al.,3 we also used “adjusted” BDI and Ham-D scores, eliminating the overlapping symptoms of loss of interest, low energy, and psychomotor retardation (loss of insight was never scored, so its deletion was irrelevant). Outlook or future orientation was assessed with Beck's 20-item self-report Hopelessness Scale.18 The 7-item Global Assessment of Recent Stress19 also was administered.
Apathy Evaluation Scale (AES):2 The psychometric properties of this scale have been well established. Clinician, informant, and self-report versions are available; the latter was used here. The scale consists of 18 items rated on a 4-point Likert-type scale ranging from 1 (“not at all”) to 4 (“a lot”). Scores range from 18 to 72; those of 38 or higher are considered to signify “clinical” apathy. Questions address the respondent's thoughts, emotions, and activities. The AES is, however, unifactorial.1 To match other scales in our battery, we used a time frame of the past week. Sample items: “I am interested in learning new things” and “When something good happens, I get excited.”
Adherence Attitudes and Outlook: The Adherence Determinants Questionnaire (ADQ)20 was designed to assess elements thought to be associated with patients' adherence to cancer control regimens. The items were modified as needed for HIV+ respondents. We used the following subscales: 1) Barriers—e.g., ”Lots of things get in the way of following my treatment plan“; 2) Intentions—e.g., ”I have made a commitment to follow my treatment plan“; 3) Interpersonal Aspects—referring to the doctor-patient relationship, as in, “The doctors act like I'm wasting their time”; and 4) Perceived Utility—e.g., “The benefits of my treatment plan outweigh any difficulty I might have in following it.” In the original scale development study and in four validation studies with a total of 480 medical patients, internal consistency reliabilities for these subscales averaged 0.67, 0.78, 0.83, and 0.75, respectively. Scores were minimally related to a measure of social desirability (r=+0.12, not significant). Self-reported general and specific adherence showed strong relationships, in all four samples, with the perceived availability of social supports and perceived absence of barriers. Each subscale has 4 to 8 items with responses ranging from 1 (“strongly disagree”) to 5 (“strongly agree”).
Neuropsychological Assessments: A neuropsychological test battery assessed the domains of attention, concentration, learning, memory, psychomotor speed, and executive function. The tests administered were the Color Trail Making Test 1 and 2,21 The Rey Auditory Verbal Learning Test,22 the Grooved Pegboard Test,23 and the Stroop Color Word Test.24 Raw scores were used as continuous measures.
Medical Measures: These measures included current CD4 cell count and HIV RNA viral load, which are both laboratory assays. Reports of current HIV symptoms such as unexplained fever or weight loss were elicited during the clinical interview with a 14-item checklist used previously at this and other sites.25
Procedure
After conducting the informed consent process and obtaining written consent for participation in this Institutional Review Board–approved study, interviewers first gathered sociodemographic information, medical history, and medication data. Neuropsychological tests were conducted next, followed by the other interviewer- administered scales; self-reports were completed last. Interviewers were pre- and postdoctoral fellows trained to conduct the psychiatric and neuropsychological evaluations; ongoing biweekly supervision of the interviewers included monitoring of audiotapes of the SCID and Ham-D.
Statistical Analyses
Scale score distributions were inspected for kurtosis and skew, and square root or log10 transformations were performed as indicated. Results of the HIV RNA assay are conventionally expressed in log10 units because of the great range, from less than 400 copies (“undetectable”) to several million. Pearson correlation coefficients were calculated for linear scale data, and t-tests were performed for group comparisons. Because of the multiple measures and comparisons entailed, we used P≤0.01 to denote statistical significance, with P>0.01 to <0.05 to indicate trend significance. All tests were two- tailed. Data sets for men and women were analyzed separately because of the marked differences in background characteristics.
RESULTS
Men's Sample
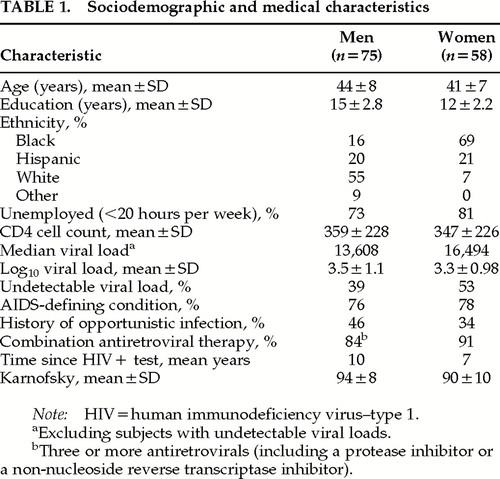
As shown in Table 1, the 75 men on average were in early middle age, with post–high school education or training, and 45% were nonwhite. Nearly three-quarters were unemployed or worked less than 20 hours per week. Their health was relatively stable. Although 84% were currently receiving three or more antiretroviral medications, most had received serial monotherapy with antivirals before the introduction of combination therapy, which may account for the relatively low proportion with undetectable viral load.
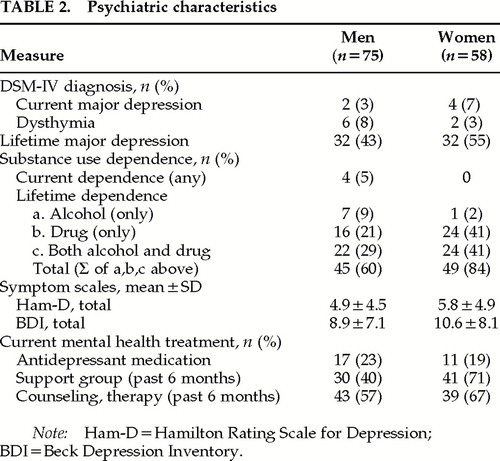
As shown in Table 2, current Axis I psychiatric disorders were rare; 3% (n=2) had a current major depression and another 8% (n=6) had dysthymia. Mean scores on the Ham-D and BDI, measuring depressive symptoms, were in the “not depressed” range, and rates for current depressive disorders were low, in contrast to the 43% rate for lifetime major depression. Similarly, only 5% had a current substance use disorder of any kind, while a total of 60% had lifetime alcohol, drug, or both alcohol and drug use disorders (not including intravenous drug use, a study exclusion criterion for men). To some extent, the cumulative lifetime rates may be inflated because this was the fourth time that the men were assessed with the SCID, and raters were not blind to prior diagnoses.
Mean score on the Apathy Evaluation Scale was 28 (SD=8.2). Eight men (11%) scored in the “clinical” range. On this fourth semiannual administration of neuropsychological tests, 9 men (12%) were outliers, defined as scoring at least 1.5 standard deviations below group means on two or more nonredundant tests. Twenty-one percent (n=16) had a potential neurocognitive confounder, including diagnosis of a learning disability (n=3), seizures (n=4), loss of consciousness >30 minutes (n=3), or CNS opportunistic infection (n=6).
Women's Sample
The 58 women were similar in age to the men, with somewhat fewer years of education (Table 1). They were substantially different with respect to ethnicity/race and education: only 7% were white, and black women outnumbered Hispanic women by 3:1. Four out of five were unemployed or working less than 20 hours a week.
Their health was stable, with their CD4 cell counts virtually the same as that of the men; 78% had an AIDS- defining condition, which also is close to the rate in the male sample. Fewer women than men (34% vs. 46%; χ2=2.95, P≤0.09) had histories of opportunistic infections; their AIDS diagnosis was more commonly based on CD4 cell counts <200 cells/mm3 at some time in the past. Ninety-one percent were taking combination antiretroviral medications, and 53% of women vs. 39% of men had undetectable viral loads. (χ2=2.9, df=1, P≤0.09).
Current depressive disorders were rare (a total of 6 [10%] had either major depression or dysthymia), but 55% had a lifetime history of major depression (Table 2). The average depression score on symptom scales was in the “not depressed” range on the Ham-D, and the mean BDI score of 10.6 was borderline (10+ is the cutoff for mild depression).
Eighty-four percent of women had a lifetime history of substance use disorder with or without alcohol dependence, as shown on Table 2, but all were currently sober, as required by study inclusion criteria. The most common drug of abuse was cocaine (n=37), followed by opioids (n=32)—these are not mutually exclusive. Ninety percent of women with lifetime major depression diagnoses also had lifetime substance use disorders.
The Apathy Evaluation Scale mean score was 29 (SD=7.0). Eight women (14%) scored in the clinical range. Twenty-six women (45%) were classified as outliers on this first administration of neuropsychological tests. Fourteen (24%) reported a potential neurocognitive confounder, including CNS opportunistic infection (n=1), special education (n=7), head injury (n=1), encephalopathy (unspecified, n=1), and seizures (n=4).
What Are the Relationships Among Apathy, Depression, and Cognitive Impairment?
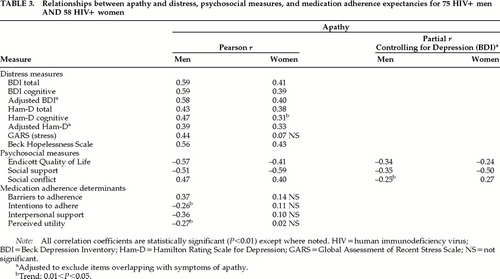
As shown in Table 3, apathy and depression measures are consistently correlated in both samples, on clinician and self-rated measures of depressive symptoms, stress, and hopelessness. Using the adjusted Ham-D and BDI scales (removing items referring to loss of interest, low energy, and psychomotor retardation, which are defining criteria for both depression and apathy), correlations are reduced but remain statistically significant. When these analyses were repeated, controlling for current use of antidepressant medication, no correlation coefficient changed by more than 0.03 and statistical significance did not change (data not shown).
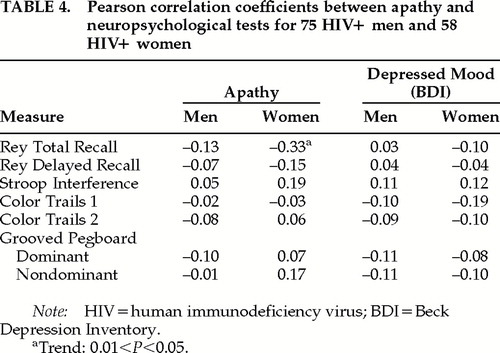
The relationship between neuropsychological test performance and apathy scores is shown in Table 4. Of the seven measures generated by the four tests, none was significantly correlated with apathy. In the women's sample, where 45% were classified as outliers on the composite neuropsychological battery, one of the seven scores was significantly correlated: less apathy was associated with better short-term memory. In neither sample were there significant correlations between measures of depression (Ham-D and BDI) and neuropsychological test scores, as seen in Table 4. As above, the analyses in Table 4 were repeated controlling for current antidepressant use, and again, results were essentially unchanged (data not shown).
Is Apathy Associated With HIV Illness Progression?
Apathy scores were uncorrelated with CD4 cell count (r= –0.05 and –0.14 for men and women, respectively) or HIV RNA viral copies (r= –0.13 and +0.11 for men and women, respectively). Men with and without undetectable viral load did not differ in terms of apathy (means of 27 and 31, respectively; t=1.9, df=72, P=0.06), nor did patients with and without an AIDS- defining condition (means of 28 and 29, respectively; t=0.68, df=73, not significant). Similarly among women, apathy scores did not differ for those with and without undetectable viral load (means of 28 and 30; t=1.12, df=51, not significant) or those with and without an AIDS diagnosis (means of 28 and 31; t=1.4, df=51, not significant). Number of current HIV symptoms similarly was uncorrelated with apathy scores (r= –0.01 for men and 0.06 for women).
Seven men and 7 women met Marin's (1993) definition of “pure apathy” (scores of 38+ on the AES and <11 on the Ham-D scale). They did not differ from the other participants in terms of markers of HIV illness progression or neuropsychological outlier status (data not shown).
What Is the Association Between Apathy and Attitudes Toward Medication Adherence?
Pearson correlation coefficients between subscales of the Adherence Determinants Questionnaire show a consistent pattern of relationship with apathy scores for men but not for women, as shown in Table 3. Apathy was positively associated with greater perceived barriers to adherence and was negatively associated with perceived interpersonal support for adherence, intentions to adhere, and perceived utility. However, when partial correlations were performed, controlling for depressive symptoms (BDI), only two correlation coefficients remained significant, and only at the trend level.
DISCUSSION
The initial findings reported by Castellon et al.14 of a significant association between apathy and neuropsychological test performance were not replicated in either of the two samples we studied. It is true that we used different measures from Castellon and colleagues both for apathy and for cognitive impairment; apathy was rated by clinicians in their study and by self-report in ours. Further, Castellon et al. studied only reaction time and working memory, whereas we evaluated executive function, memory, and psychomotor speed, which are the domains most consistently shown to be affected by HIV infection.8 In addition, our samples included a greater proportion of subjects with late-stage HIV illness. Also, mean scores on both depressive symptoms and apathy were higher, and standard deviations were larger, in Castellon's sample compared with ours. This might imply that our failure to find a relationship between apathy and neuropsychological tests represents a “false negative” finding due to attenuation in score range. However, since we did find significant and robust correlations between apathy and depressive severity, this explanation seems unlikely. Overall, the disparity between these findings requires reconsideration of the relationship between apathy (at least as measured here) and cognitive impairment in HIV infection.
Apathy was consistently associated with multiple measures of depression, even when overlapping items were deleted from the depression measures, among men and women most of whom had AIDS-defining conditions but not syndromal depressive disorders. Notably, with only one exception, apathy was unrelated to any of the neuropsychological tests that were administered to measure executive function, memory, and psychomotor speed in our two separately analyzed samples. It is, however, possible that the relatively limited focus of the cognitive evaluation in this study may have contributed to the lack of an identified relationship.
Our results are not in accord with Marin's finding3 of the discriminability of depression and apathy once patients with current major depression are removed from consideration. In both of our samples, less than 10% had current major depression and/or dysthymia, and the removal of these cases from data analyses did not alter the observed relationships. Furthermore, levels of depression were low overall; the mean scores of measures of depressive symptoms were in the “not depressed” range for men and on the border of “mild symptoms” for one of the two scales for women. (It should be noted that about 20% of subjects in each sample were taking antidepressant medication, which presumably ameliorated depressive symptoms and disorders that otherwise might have been diagnosed.) Although the constructs of depression and apathy are related in our samples, they are not redundant, since at best each accounts for only 25% of the variance in the other.
Although we observed an association between patient expectancies regarding medication adherence and levels of apathy in the men's sample, the relationship was no longer significant when we controlled for depression, which appears to be the predominant determinant of these expectancies. There was no association between apathy and expectancies regarding medication adherence in the women's sample even without controlling for depression. While these cumulative results are not entirely clear, it seems unlikely that the concept of apathy serves a useful role in understanding a person's appraisal of factors associated with medication adherence.
CONCLUSION
Apathy was associated with depressive symptoms but not with cognitive impairment in separately analyzed samples of HIV+ men and women who, despite major sociodemographic differences, performed similarly with respect to the relationships among these three dimensions. These findings raise questions about the presumed association between apathy and neurocognitive disorders in HIV (i.e., minor cognitive motor disorder and HIV-associated dementia). Perhaps apathy is best assessed by observer rating rather than self-rating. Alternatively, perhaps only certain cognitive domains are associated with apathy—such as working memory, which is one of several frontal lobe functions not measured in this study. The current findings underscore the need to investigate the association between self-ratings and observer ratings of apathy and to further assess the association of apathy with specific cognitive domains.
ACKNOWLEDGMENTS
This work was partially supported by National Institute of Mental Health Grant R01 MH42277.
 |
 |
 |
 |
1 Marin RS: Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals 1997; 27:30–33Crossref, Google Scholar
2 Marin RS, Biedrzycki R, Firinciogullari S: Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991; 38:143–162Crossref, Medline, Google Scholar
3 Marin RS, Firinciogullari, Biedrzycki R: The sources of convergence between measures of apathy and depression. J Affect Disord 1993; 28:117–124Crossref, Medline, Google Scholar
4 Marin RS: Apathy and related disorders of diminished motivation, in Review of Psychiatry, vol 15, edited by Dickstein LJ, Riba MB, Oldham JM. Washington, DC, American Psychiatric Press, 1996, pp 205–242Google Scholar
5 Marin RS: Apathy—who cares? An introduction to apathy and related disorders of diminished motivation. Psychiatric Annals 1997; 27:18–23Crossref, Google Scholar
6 Levy ML, Cummings JL, Fairbanks LA, et al: Apathy is not depression. J Neuropsychiatry Clin Neurosci 1998; 10:314–319Link, Google Scholar
7 Rabkin JG: Prevalence of psychiatric disorders in HIV illness. Int J Psychiatry 1996; 8:157–166Google Scholar
8 Hinkin C, Castellon W, Van Gorp W, et al: Neuropsychological features of HIV disease, in Practitioner's Guide to the Neuropsychiatry of AIDS, edited by van Gorp W, Buckingham S. New York, Guilford, 1998, pp 1–41Google Scholar
9 White DA, Heaton R, Monsch AU: Critical review: neuropsychological studies of asymptomatic human immunodeficiency virus-type-1 infected individuals. J Int Neuropsychol Soc 1995; 1:304–315Crossref, Medline, Google Scholar
10 Stern Y, Marder K, Bell K, et al: Multidisciplinary baseline assessment of homosexual men with and without HIV infection. Arch Gen Psychiatry 1991; 48:131–138Crossref, Medline, Google Scholar
11 Bornstein RA, Pace P, Rosenberger P, et al: Depression and neuropsychological performance in asymptomatic HIV infection. Am J Psychiatry 1993; 150:922–927Crossref, Medline, Google Scholar
12 Grant I, Olshen R, Atkinson JH, et al: Depressed mood does not explain neuropsychological deficits in HIV-infected persons. Neuropsychology 1993, 7:53–61Google Scholar
13 Nomenclature and research case definitions for neurologic manifestations of HIV-1 infection: report of a working group of the American Academy of Neurology AIDS Task Force. Neurology 1991; 41:778–785Crossref, Medline, Google Scholar
14 Castellon S, Hinkin CH, Wood S, et al: Apathy, depression, and cognitive performance in HIV-1 infection. J Neuropsychiatry Clin Neurosci 1998; 10:320–329Link, Google Scholar
15 Spitzer RL, Williams J, Gibbon M, et al: Structured Clinical Interview for DSM-IV. Washington DC, American Psychiatric Press, 1995Google Scholar
16 Williams JBW: A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry 1988; 45:742–747Crossref, Medline, Google Scholar
17 Beck AT, Ward CH, Mendelson M, et al: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:53–63Crossref, Google Scholar
18 Beck AT, Weissman A, Lester D, et al: The measurement of pessimism: the Hopelessness Scale. J Consult Clin Psychol 1974; 42:861–865Crossref, Medline, Google Scholar
19 Linn MW: A global assessment of recent stress scale (GARS). Int J Psychiatry Med 1985–1986; 15:47–59Google Scholar
20 DiMatteo MR, Hays R, Gritz E, et al: Patient adherence to cancer control regimens: scale development and initial validation. Psychological Assessment 1993; 5:102–112Crossref, Google Scholar
21 D'Elia LF, Satz P, Uchiyama C, et al: Color Trails Test. Odessa, FL, Psychological Assessment Resources, 1996Google Scholar
22 Lezak MD: Neuropsychological Assessment, 3rd edition. New York, Oxford University Press, 1995Google Scholar
23 Matthews CG, Klove H: Instruction manual for the adult neuropsychology test battery. Madison, WI, University of Wisconsin Medical School, 1964Google Scholar
24 Golden JC: Stroop Color and Word Test. Chicago, Stoetling, 1978Google Scholar
25 Rabkin JG, Ferrando S, Jacobsberg L, et al: Prevalence of Axis I psychiatric disorders in an AIDS cohort: a controlled study. Compr Psychiatry 1997; 38:146–154Crossref, Medline, Google Scholar



