Do Testosterone Levels Relate to Aggression in Elderly Men With Dementia?
Abstract
The aim of this study was to determine if testosterone and estrogen levels correlate with aggression in older men with dementia. Plasma total and free testosterone and estrogen levels and scores for behavioral disturbances, in particular aggression, were measured in 50 elderly males who had a diagnosis of dementia. Aggression was analyzed separately from agitation. Pearson correlations were calculated to determine the association between testosterone and estrogen and aggression. Linear regression analyses determined the influence of hormone levels on aggression, controlling for age, medical burden, and dementia severity. Free testosterone levels showed significant positive correlations with measures of aggression. Estrogen levels showed significant negative correlations with measures of aggression.
Several reports suggest that testosterone engenders aggression in males. Studies in prisoners have found evidence of a positive correlation between testosterone and aggression.1,2 Violent male offenders show substantially higher plasma testosterone levels than less violent individuals.1,2 Similar results were obtained from a volunteer male sample divided according to the presence or absence of aggression when drinking alcohol3 or while playing a competitive sport.4
The relationship between testosterone and aggression in elderly men with dementia has not been systematically investigated. Case studies have reported the efficacy of estrogen treatment on aggressive behavior in 2 men with dementia and in 1 with traumatic brain injury.5,6 A randomized, double-blind placebo-controlled trial found that estrogen therapy was associated with lower aggression in 7 women and 1 man with dementia in a long-term care facility.7 Other studies, totaling fewer than 10 patients, have reported that the administration of medroxyprogesterone acetate, estrogen, and leuprolide acetate decreases aggression and sexually aberrant behavior in men with and without dementia.8–11 We have reported a significant correlation between testosterone levels and physical aggression in 13 men with dementia.12
In this study, we hypothesized that testosterone levels are related specifically to aggression (as distinguished from agitation) and that aggression is specifically and inversely related to estrogen levels.
METHODS
Subjects
All male patients who were living at home or in nursing homes who presented to the Houston Veterans Affair Medical Center Outpatient Geriatric Psychiatry Clinic with a DSM-IV diagnosis of dementia were screened for inclusion in the study. Patients and their next of kin signed an Institutional Review Board–approved informed consent document. Patients were excluded if liver enzymes or serum creatinine were twice the upper limit of normal and if they were taking any medications that interfere with testosterone levels (i.e., cimetidine, spironolactone, ketoconazole, leuprolide acetate, or estrogen). Patients who had unstable medical illnesses or who were diagnosed with other psychiatric illnesses that may have contributed to their behavior, such as bipolar disorder, schizophrenia, or posttraumatic stress disorder, were also excluded.
Assessments
Subjects were rated for degree of aggression/agitation over the 2 weeks prior to the interview by use of the Overt Aggression Scale (OAS),13 the Overt Agitation Severity Scale (OASS),14 and the Cohen-Mansfield Agitation Inventory (CMAI).15 The CMAI measures three factors of agitation: factor 1, physically aggressive behavior (CMAI fac1), factor 2, physically nonaggressive behavior (CMAI fac2), and factor 3, verbally aggressive behavior (CMAI fac3). Information for completing the assessments was gathered from observation and by interview with the patient, caregiver, and family.
Subjects' general psychiatric symptoms were rated with the Behavioral Pathology in Alzheimer's Disease Rating Scale (BEHAVE-AD)16 or the Neuropsychiatric Inventory (NPI).17 The BEHAVE-AD was replaced by the NPI after the first 28 subjects were enrolled. Cognitive status was rated by the Mini-Mental State Examination (MMSE)18 and the Dementia Rating Scale (DRS).19 Medical burden was assessed with the Cumulative Illness Rating Scale (CIRS).20 Diagnosis of dementia was confirmed by a board-certified psychiatrist after clinical examination and review of the patient's record and history obtained from the family and friends. A variety of demographic and clinical characteristics were collected, including age, race, marital status, type and duration of dementia, current medications, and medical diagnoses.
Hormone Levels
Hormone levels were measured by obtaining three blood samples 15 minutes apart between 7:00 and 10:00 a.m. All blood samples were taken on the morning of the above ratings. The three serum samples were pooled and stored at –20° C. Taking three blood samples provides a more accurate assessment of testosterone levels because testosterone is secreted in a pulsatile fashion. Total testosterone and estradiol levels were measured in duplicate by using a Diagnostic Systems Laboratories radioimmunoassay kit. Free testosterone levels were measured in duplicate by using a Diagnostic Products Corporation radioimmunoassay kit. The free form of testosterone gives a more accurate measure of testosterone availability at the cerebral level. All samples were processed in one assay by a technician blinded to patients and ratings. Values that fell ≥ one standard deviation above or below standard laboratory values were verified by repeating the assay.
Statistical Analysis
The focus of the research was on the relationship of physically aggressive behavior and hormone levels. Measures of aggression are typically combined with measures of agitation;21–23 however, we were interested in aggression alone. Therefore we combined the physical aggression and verbal aggression subscales of the CMAI, the OAS total, the aggression subscale of the NPI, and the aggression subscale of the BEHAVE-AD in a latent-variable model to estimate individual scores on the latent variable representing aggression. Within the latent-variable framework, we assume that individuals have some latent amount of aggression that is indicated by the scores on the five measures of aggression.24,25 One advantage of this approach was our ability to combine only measures of aggression while excluding measures of agitation. In addition, this approach reduced the number of analyses we conducted to assess the relationship of hormone levels to the various measures of aggression, thereby maximizing power. Because of the potential for unstable factor loadings in small samples, we constrained the relationship between each measure and the latent variable to be equal.24,25 In a simulation study, Marsh and Hau26 found that 98% of the factor models reached proper solutions when the N was equal to 50 and there were 6 indicators per factor. After determining that the model adequately fit the data by assessing whether data from our study fit a model with one latent variable and five measures of aggression, we used the estimated individual scores on the latent variable as the aggression score. We also evaluated the relationship of hormone levels to each of the subscales separately.
Descriptive statistics were used to report demographic and clinical data. Correlational analyses were conducted to assess if hormone levels correlated with behavioral and cognitive measures. Linear regression analyses were made to determine the influence of hormone levels on aggression, with age, medical burden and dementia severity controlled. Power analysis determined that a sample size of 48 was necessary to detect a population R2 as small as 0.2, if the alpha level was set at 0.05 and the power level at 80%.
RESULTS
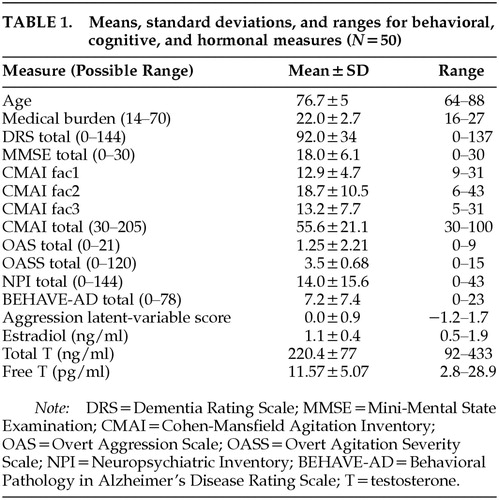
A total of 50 patients met criteria and agreed to participate in the study. Ten patients met criteria but refused to participate. The average age was 76.7±5 years (means and standard deviations are reported). Fifty-eight percent were Caucasian, 30% African American, and 12% Hispanic. Seventy-six percent were living at home, 12% in personal care homes, and 12% in nursing homes. Sixty percent (31 patients) had a diagnosis of Alzheimer's dementia, 28% (14 patients) had a diagnosis of vascular dementia, 6% (3 patients) had a diagnosis of mixed dementia, and the remaining 4% (2 patients) had alcohol-induced persisting dementia. The average MMSE score was 18±6, and the average DRS score was 92.2±34. Table 1 shows the means, standard deviations, and ranges for the behavioral, cognitive, and hormone measures. The total CMAI score for the subject population is similar to that reported in other studies investigating behavioral dyscontrol in community-dwelling individuals with dementia.23,27
Fit of the data to the latent-variable model measuring aggression was good and demonstrated that the model adequately represented the data, as evidenced by the chi-square test of goodness of fit (χ2=8.0, df=7, P=0.33) and the root mean square error of approximation (0.06).24,25 The reliability of the latent variable was 0.90, and the proportion of variance captured by the latent variable relative to the amount of variance due to measurement error, or the average variance extracted, was 0.69.28
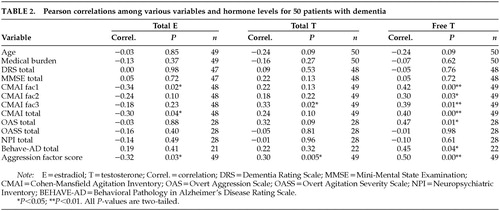
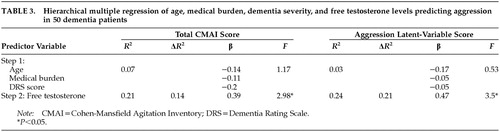
Table 2 shows the correlation coefficient for hormone levels and measures of cognition and behavior. Significant positive correlations were found between free T levels and CMAI fac1, CMAI fac2, CMAI fac3, CMAI total, OAS, and aggression latent-variable scores. Estrogen levels show a negative significant correlation with CMAI fac1 and total CMAI. Multiple regression equations were computed for total CMAI score and for the aggression latent-variable scores. Age, medical burden (CIRS), and dementia severity (DRS score) were entered on the first step, and free testosterone was entered on the second step. Table 3 lists the results from the regression analysis. Free testosterone alone accounts for 14% of the variance in total CMAI score and 21% of the variance in aggression latent-variable score.
DISCUSSION
We found levels of testosterone to be positively correlated and levels of estrogen to be negatively correlated with the specific behavior of physical aggression in elderly men with dementia, regardless of the type of dementia. Free testosterone accounts for 21% of the variance in the aggression latent-variable score when controlling for age, dementia severity, and medical illness. The biologic plausibility of the inverse relationship between estrogen and testosterone, together with the finding of specificity that neither testosterone nor estrogen level was associated with other behavior disturbances such as psychomotor agitation (OASS), psychosis (paranoia-hallucination subscale of NPI or delusion-hallucination subscales of BEHAVE-AD), depression, or mania (affect subscale of NPI or depression-euphoria subscale of BEHAVE-AD) adds strength to the finding. When these findings are evaluated in conjunction with the double-blind study by Kyomen et al.7 showing a temporal relationship between estrogen treatment and decreased aggression, an argument can be made for an important role of testosterone and estrogen in aggression in elderly male patients with dementia.
Often physicians and other healthcare professionals group together many diverse behavioral disturbances as “agitation.” Such a vague, global concept hinders clinical quantification, obscures underlying disorders, and can result in inappropriate use of pharmacotherapy or physical restraints. Aggression is defined as recurrent violent events, either verbal or physical in nature, that are out of proportion to the precipitating stress or provocation and that stem from organic etiologies. Agitation is excessive and/or inappropriate verbal, vocal, or motor activity, which may or may not be voluntary. In this study we show that free testosterone levels are correlated specifically with aggression and not with agitation.
This study measured plasma free testosterone rather than cerebrospinal fluid (CSF) testosterone. Because of its lipophilic nature, testosterone easily passes through the blood–brain barrier in free form (not bound to protein). At the cerebral level, steroid hormones originating from the periphery influence the function of nerve cells dispersed throughout the body. The best example of this influence is the neurons that secrete hypophysiotrophic factors stimulating the production of pituitary hormones such as ACTH and gonadotropins. These hormones are subjected to regulation by the corresponding steroid hormones by a feedback mechanism.
Testosterone and estrogen receptors are primarily located in the limbic areas of the brain. Many studies in animals and humans have found that rage reactions occur with stimulation of limbic structures but not as the result of stimulation of the neocortex.29,30 Phylogenetically and histologically, the limbic gray matter is clearly more primitive than the other cortical areas. Individuals with dementia have diminished higher cortical function that may lead to a decrease in the psychosocial or environmental influences on behavior. Therefore, a demented individual may be more influenced by primitive or limbic impulses and may lack the inhibitions of learned psychosocial behaviors. This explanation is consistent with the hypothesis that deeper limbic structures contain the template for aggressive behavior but that higher centers, when intact, maintain control of aggression.
Testosterone and estrogen levels appear to be important determinants of the complex behavior termed aggression. However, 79% of the variance of aggression is unaccounted for in our regression model. Clearly other biologic or psychosocial determinants exist and need to be explored. The role of other neurotransmitters such as serotonin, dopamine, norepinephrine, and arginine vasopressin should also be explored. Aggression is an end product of brain function, and although modified, enhanced, or diminished by environmental factors, it has as its nucleus a complex interaction of anatomic, chemical, and physiologic determinants.
Future studies should include larger sample sizes of individuals specifically selected for physically aggressive behavior problems (unrelated to psychosis) to assess adequately the aggression–testosterone relationship with subgroups of aggressive patients with different types of dementia. Future investigations could also measure testosterone levels in CSF to determine if a relationship exists between CSF concentrations of testosterone and aggression.
The results of this study offer new data to aid in the understanding of aggression in elderly men with dementia. We believe that all agitation/aggression in dementia is not the same, and suggest that different behaviors warrant specific treatments. For example, agitated patients with psychotic features require antipsychotics, whereas those with agitated depression do best with antidepressant treatment and those with physical aggression may need androgen-lowering agents. Further research on specific treatments for specific agitated behaviors in elderly patients with dementia is greatly needed. In addition, other factors associated with aggression in patients with dementia should be investigated, including premorbid personality, environmental triggers, aggressor–victim relationship, frontal lobe pathology, and acute medical conditions causing delirium.
The limitations of this study include an all-male sample of veterans in an outpatient geropsychiatric clinic. Nonetheless, our data indicate a specific association between aggressive behavior and testosterone and suggest that trials to test novel treatment approaches for physically aggressive patients (e.g., lowering testosterone levels) should be undertaken. Our nation's population is aging, the incidence of dementia is increasing, and the need to find safe and effective treatments for the behavioral disturbances associated with dementia is growing.
ACKNOWLEDGMENTS
This work was supported by pilot funds from the Baylor College of Medicine Alzheimer's Disease Research Center and by the National Brookdale Fellowship. An abstract was previously presented in a poster session at the 13th Annual Meeting of the American Association of Geriatric Psychiatry, Miami, FL, March 12–15, 2000, and at the Second International Congress on Hormones, Brain and Neuropsychopharmacology, Rhodes, Greece, July 15–19, 2000.
 |
 |
 |
1 Kreuz LE, Rose RM: Assessment of aggressive behavior and plasma testosterone in a young criminal population. Psychosom Med 1972; 34:321-332Crossref, Medline, Google Scholar
2 Dabbs JM, Frady RL, Carr TS, et al: Saliva testosterone and criminal violence in young adult prison inmates. Psychosom Med 1987; 49:174-182Crossref, Medline, Google Scholar
3 Lindman R, Jarvinen P, Vidjeskog J: Verbal interactions of aggressively and nonaggressively predisposed males in a drinking situation. Aggressive Behavior 1987; 13:187-196Crossref, Google Scholar
4 Scaramella TJ, Brown WA: Serum testosterone and aggressiveness in hockey players. Psychosom Med 1978; 40:262-265Crossref, Medline, Google Scholar
5 Kyomen HH, Nobel KW, Wei JY: The use of estrogen to decrease aggressive physical behavior in elderly men with dementia. J Am Geriatr Soc 1991; 39:1110-1112Crossref, Medline, Google Scholar
6 Arnold SE: Estrogen for refractory aggression after traumatic brain injury (letter). Am J Psychiatry 1993; 150:1564-1565Medline, Google Scholar
7 Kyomen HK, Satlin A, Hennen J, et al: Estrogen therapy and aggressive behavior in elderly patients with moderate-to-severe dementia. Am J Geriatr Psychiatry 1999; 7:339-348Medline, Google Scholar
8 Cooper A: Medroxyprogesterone acetate (MPA) treatment of sexual acting out in men suffering from dementia. J Clin Psychiatry 1987; 48:368-370Medline, Google Scholar
9 Ross L, Bland W, Ruskin P, et al: Antiandrogen treatment of aberrant sexual activity (letter). Am J Psychiatry 1987; 144:1511Medline, Google Scholar
10 Weiner M, Denke M, Williams K, et al: Intramuscular medroxyprogesterone acetate for sexual aggression in elderly men (letter). Lancet 1987; 339(8801):1121-1122Google Scholar
11 Shelton PS, Brooks VG: Estrogen for dementia-related aggression in elderly men. Ann Pharmacother 1999; 33:808-812Crossref, Medline, Google Scholar
12 Orengo CA, Kunik ME, Ghusn H, et al: Correlation of testosterone with aggression in demented elderly men. J Nerv Ment Dis 1997;185:349-351Google Scholar
13 Silver JM, Yudofsky SC: The Overt Aggression Scale: overview and guiding principles. J Neuropsychiatry Clin Neurosci 1991; 3(2, suppl 1):S22-S29Google Scholar
14 Yudofsky SC, Kopecky H, Kunik ME, et al: The Overt Agitation Severity Scale for the objective rating of agitation. J Neuropsychiatry Clin Neurosci 1997; 9:541-548Link, Google Scholar
15 Cohen-Mansfield J, Marx MS, Rosenthal AS: A description of agitation in a nursing home. J Gerontol 1989; 44:M77-M84Google Scholar
16 Reisberg B, Borenstein J, Salob SP, et al: Behavioral symptoms in Alzheimer's disease: phenomenology and treatment. J Clin Psychiatry 1987; 48(suppl 5):9-15Google Scholar
17 Cummings JL: The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997; 48(suppl 6):S10-S16Google Scholar
18 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
19 Vitaliano PP, Breen AR, Russo J: The clinical utility of the Dementia Rating Scale for assessing Alzheimer patients. Journal of Chronic Diseases 1984; 37:743-753Crossref, Medline, Google Scholar
20 Linn BS, Linn MW, Gurel L: Cumulative Illness Rating Scale. J Am Geriatr Soc 1968; 16:622-626Crossref, Medline, Google Scholar
21 Cohen-Mansfield J, Billig N: Agitated behaviors in the elderly, I: a conceptual review. J Am Geriatr Soc 1986; 34:711-721Crossref, Medline, Google Scholar
22 Harwood DG, Ownby RL, Barker WW, et al: The Behavioral Pathology in Alzheimer's Disease Scale (BEHAVE-AD): factor structure among community-dwelling Alzheimer's disease patients. Int J Geriatr Psychiatry 1998; 13:793-800Crossref, Medline, Google Scholar
23 Weiner MF, Koss E, Patterson M, et al: A comparison of the Cohen-Mansfield Agitation Inventory with the CERAD Behavioral Rating Scale for Dementia in community-dwelling persons with Alzheimer's disease. J Pscychiatr Res 1998; 32:347-351Crossref, Medline, Google Scholar
24 Kline RB: Principles and Practice of Structural Equation Modeling. New York, Guilford, 1998Google Scholar
25 Byrne BM: Structural Equation Modeling With EQS and EQS/Windows: Basic Concepts, Applications, and Programming. Thousand Oaks, CA, Sage, 1994Google Scholar
26 Marsh HW, Hau KT: Confirmatory factor analysis: strategies for small sample sizes, in Statistical Strategies for Small Sample Research, edited by Hoyle RH. Thousand Oaks, CA, Sage, 1999Google Scholar
27 Cohen-Mansfield J, Werner P: Longitudinal changes in behavioral problems in old age: a study of a day care population. J Gerontol A Biol Sci Med Sci 1998: 53:M65-71Google Scholar
28 Fornell C, Larcker DF: Evaluating structural equation models with unobservable variables and measurement error. Journal of Marketing Research 1981; 18:39-50Crossref, Google Scholar
29 Heath RG, Dempsey GW, Fontana CJ: Cerebellar stimulation: effects on septal region, hippocampus, and amygdala. Biol Psychiatry 1978; 13:501-529Medline, Google Scholar
30 Piacente GJ: Aggression. Psychiatr Clin North Am 1986; 9:329-339Crossref, Medline, Google Scholar



