Divalproex in the Management of Neuropsychiatric Complications of Remote Acquired Brain Injury
Abstract
A retrospective chart review was conducted on 11 patients with a remote history of acquired brain injury (ABI) referred for psychiatric treatment who were treated with divalproex sodium alone or in combination with other psychotropic medications. The patients were highly heterogeneous. They had a variety of psychiatric symptoms and frequently received concomitant psychotropic medications. The mean daily dose of divalproex was 1,818±791 mg/day, serum valproic acid level 85.6±29.6 μg/ml. Mean Clinical Global Impression improvement score was 1.9±0.5. This is the largest postacute case series reported. It demonstrates that divalproex sodium is well tolerated and effective in reducing a broad range of neurobehavioral symptoms in psychiatric patients with a remote history of ABI.
Acquired brain injury (ABI) may be complicated by neuropsychiatric syndromes such as depression, mania, psychosis, and disinhibition.1 Patients experiencing these symptoms may be referred to mental health systems that lack the expertise or resources to identify and manage the complex array of neurobehavioral factors.2 In patients with preexisting psychiatric disorders, ABI may change the clinical presentation and treatment response of their illness.3
Divalproex sodium, an anticonvulsant with mood-stabilizing properties, has demonstrated effectiveness in treating a wide variety of primary psychiatric disorders, in addition to those induced by ABI and degenerative dementias,4 and is emerging as a safe and effective treatment for ABI-induced agitation in acute and sub-acute rehabilitation settings.5,6 The present study sought to examine the efficacy of divalproex sodium in managing neuropsychiatric disturbances in community-dwelling psychiatric patients with a remote history of ABI.
METHODS
A retrospective chart review was conducted on 11 patients referred to an acute psychiatric inpatient/partial hospital program or neuropsychiatry clinic with a history of ABI. The history of ABI was known at the time of referral in only 3 cases; in the other 8, ABI was discovered at the time of initial evaluation. Acquired brain injury was defined as any of the following: cerebrovascular accident; single head trauma resulting in altered consciousness; or repetitive head trauma due to physical abuse resulting in altered consciousness. All selected patients were treated with divalproex sodium alone or in combination with other psychotropic medications. Divalproex was titrated to clinical response. A reviewer (T.J.H.) abstracted clinical information regarding demographics, age at time of injury, age at onset of psychiatric disturbance, concomitant medications, daily divalproex dose, and serum valproate levels. The rater also assessed treatment response as documented in the chart, using the Clinical Global Impression Improvement Scale. This seven-point scale rates overall improvement from initial treatment with values from 1 (very much improved) through 4 (no change) to 7 (very much worse).
RESULTS
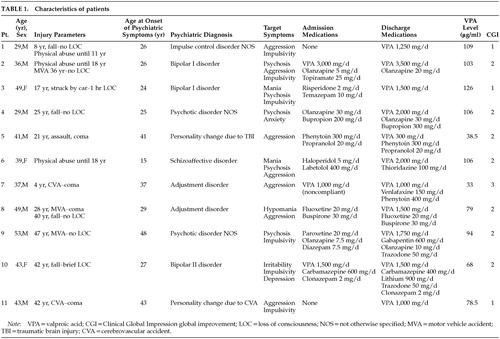
Patient demographics, injury parameters, and concomitant medications are presented in Table 1. The sample consisted of 8 males and 3 females with a mean age of 40.7±7.8 years. The mean age at onset of psychiatric disturbance was 30.9±10 years. All patients were functionally independent and living in the community. Psychiatric diagnoses included bipolar disorder (n=3), psychotic disorder not otherwise specified (n=2), personality change due to acquired brain injury (n=2), adjustment disorder (n=2), schizoaffective disorder (n=1), and impulse control disorder not otherwise specified (n=1). Four patients (36.4%) had concomitant substance abuse disorders. Symptoms upon initial evaluation included physical and verbal aggression (n=7), psychosis (n=6), impulsivity (n=5), elevated mood (n=4), and depression (n=1). Seven patients were injured in accidents; 3 were victims of physical abuse with or without additional accidents; 2 suffered cerebrovascular accidents; and 1 was injured in an assault. The age at time of injury ranged from 4 to 47 years. SPECT scans obtained as part of each patient's clinical evaluation demonstrated reduced orbitofrontal perfusion in all patients and reduced temporal perfusion in 72.3% of patients.
The mean daily dose of divalproex was 1,818±791 mg/day, with a mean serum valproic acid level of 85.6±29.6 μg/ml. Mean CGI improvement score was 1.9±0.5. Three patients were rated as extremely improved, 7 patients were much improved, and 1 patient was minimally improved. There was a negative correlation between serum valproate level and CGI improvement score (r=–0.73, P<0.01). This finding suggests a possible association between higher serum levels and better response, but the small sample size limits the power of the study. Patients all tolerated their medication regimens without adverse effects.
DISCUSSION
This study is limited by its uncontrolled retrospective design, small sample size, and patient heterogeneity. Injury mechanisms ranged from repetitive physical abuse to spontaneous subarachnoid hemorrhage. The presenting psychiatric symptoms varied considerably, and patients were receiving a wide range of concomitant psychoactive medications. Despite the diagnostic heterogeneity of our sample, aggression, psychosis, and impulsivity, either alone or in combination, were observed in all patients. Moreover, all of our patients had evidence of orbitofrontal damage and nearly three-quarters demonstrated temporal lobe hypoperfusion.
ABI frequently causes persistent changes in personality, emotion, and impulse control that may contribute to family distress and poor longitudinal outcomes.7 The mechanism mediating these neuropsychiatric complications of ABI is not well understood. Orbitofrontal and temporal basal polar lesions are associated with behavioral disturbances in both traumatic brain injury (TBI) and stroke.8,9 The orbitofrontal cortex is the only cortical structure with direct efferent connections to the hypothalamus, amygdala, and brainstem biogenic amine nuclei. Thus, this region may play a vital role in mediating arousal and instinctive behaviors, in particular aggression and impulsivity.10
Jorge et al.11 have suggested that orbitofrontal damage may induce subictal kindling due to denervation hypersensitivity in limbic structures. This kindling could enhance dopaminergic mesocorticolimbic transmission, which may in turn contribute to manic, disinhibited, and psychotic states. A common presentation in such cases is a dysphoric mixed bipolar syndrome characterized by dysphoria, irritability, and aggression.12,13 Our patients suffered from a wide variety of dopamine-dependent symptoms, including mania, aggression, and psychosis, and all demonstrated evidence of orbitofrontal damage on cerebral SPECT scans. These findings are consistent with the hypothesis of hyperdopaminergic states resulting from ABI-induced kindling. Moreover, the wide range of symptoms that appeared to improve in our sample suggests an antikindling rather than a primary antimanic therapeutic effect of divalproex.
Carbamazepine and valproic acid are effective mood stabilizers that inhibit limbic kindling in animal models.14 Both medications have been used effectively in managing brain-injured patients in the acute neurorehabilitation setting.6,15 Divalproex sodium, however, may be superior to carbamazepine with respect to cognitive side effects in brain-injured patients.16,17 Single and multiple case reports cite its effectiveness in reducing agitation, aggression, and affective lability in patients with TBI,18,19 epilepsy,20 anoxia, and dementia.21 Total daily dosages of 750–1,500 mg with serum levels of 50–110 μg/ml were described in these reports. In a retrospective study of 29 acutely brain-injured patients, Chatham Showalter and Netsky Kimmel6 found that a mean dosage of 1,250 mg/day led to rapid response within 1 week, a time frame consistent with our experience.
Agitation in acutely brain-injured patients has been conceptualized as a subtype of delirium in coma-emerging patients.22 Our postacute, community-dwelling patients represent a clinically distinct population from those in the Chatham Showalter series. Rather than demonstrating symptoms of coma-emerging delirium, our patients were experiencing symptoms consistent with a limbic kindling model of psychopathology. This is the largest case series reporting the use of divalproex sodium in managing postacute neurobehavioral complications of ABI and complements Chatham Showalter and colleague's acute care study. None of our patients experienced adverse side effects or significant drug–drug interactions. The mean dosages of divalproex and serum levels in our patients were substantially higher than those described in previous reports.
Divalproex sodium, either alone or in combination with other psychotropic medications, appears to be well-tolerated and effective in reducing a broad range of neurobehavioral symptoms in community-dwelling patients with a remote history of ABI. We suggest that the antikindling effects rather than a specific antimanic effect of divalproex is responsible for its broad range of benefits, and that limbic kindling may be responsible for a wide spectrum of ABI-induced psychopathology. The therapeutic effect may be dose-dependent, suggesting that the use of higher doses may be warranted in patients who fail to respond at lower serum levels. These preliminary findings await replication in larger, prospective trials.
 |
1 Van Reekum R, Cohen T, Wong J: Can traumatic brain injury cause psychiatric disorders? J Neuropsychiatry Clin Neurosci 2000; 12:316-327Link, Google Scholar
2 McAllister TW: Evaluation of brain injury-related behavioral disturbances in community mental health centers. Community Ment Health J 1997; 33:341-364Crossref, Medline, Google Scholar
3 Ross ED, Rush AJ: Diagnosis and neuroanatomical correlates of depression in brain-damaged patients. Arch Gen Psychiatry 1981; 38:1344-1354Crossref, Medline, Google Scholar
4 Davis LL, Ryan W, Adinoff B, et al: Comprehensive review of the psychiatric uses of valproate. J Clin Psychopharmacology 2000; 20:1S-17SCrossref, Medline, Google Scholar
5 Wroblewski BA, Joseph AB, Kupfer J, et al: Effectiveness of valproic acid on destructive and aggressive behaviours in patients with acquired brain injury. Brain Inj 1997; 11:37-47Crossref, Medline, Google Scholar
6 Chatham Showalter PE, Netsky Kimmel D: Agitated symptom response to divalproex following acute brain injury. J Neuropsychiatry Clin Neurosci 2000; 12:395-397Link, Google Scholar
7 Gleckman AD, Brill S: The impact of brain injury on family functioning: implications for subacute rehabilitation programmes. Brain Inj 1995; 9:385-393Crossref, Medline, Google Scholar
8 Robinson RG, Boston JD, Starkstein SE, et al: Comparison of mania and depression after brain injury: causal factors. Am J Psychiatry 1988; 145:172-178Crossref, Medline, Google Scholar
9 Starkstein SE, Mayberg HS, Berthier ML, et al: Secondary mania: neuroradiological and metabolic findings. Ann Neurol 1990; 27:652-659Crossref, Medline, Google Scholar
10 Starkstein SE, Robinson RG: Mechanism of disinhibition after brain lesions. J Nerv Ment Dis 1997; 185:108-114Crossref, Medline, Google Scholar
11 Jorge RE, Robinson RG, Starkstein SE, et al: Secondary mania following traumatic brain injury. Am J Psychiatry 1993; 150:916-921Crossref, Medline, Google Scholar
12 Shukla S, Cook BL, Mukherjee S, et al: Mania following head trauma. Am J Psychiatry 1987; 144:93-96Crossref, Medline, Google Scholar
13 Zwil AS, McAllister TW, Raimo E: The expression of bipolar affective disorders in brain-injured patients. Int J Psychiatry Med 1992; 22:377-395Crossref, Medline, Google Scholar
14 Post RM, Weiss SR: A speculative model of affective illness cyclicity based on patterns of drug tolerance observed in amygdala-kindled seizures. Mol Neurobiol 1996; 13:33-60Crossref, Medline, Google Scholar
15 Chatham Showalter PE: Carbamazepine for combativeness in acute traumatic brain injury. J Neuropsychiatry Clin Neurosci 1996; 8:96-99Link, Google Scholar
16 Dikmen SS, Machamer JE, Winn HR, et al: Neuropsychological effects of valproate in traumatic brain injury: a randomized trial. Neurology 2000; 54:895-902Crossref, Medline, Google Scholar
17 Geracioti TD: Valproic acid treatment of episodic explosiveness related to brain injury. J Clin Psychiatry 1994; 55:416-417Medline, Google Scholar
18 Pope HG, McElroy SL, Satlin A, et al: Head injury, bipolar disorder, and response to valproate. Compr Psychiatry 1988; 29:34-38Crossref, Medline, Google Scholar
19 Monji A, Yoshida I, Koga H, et al: Brain injury induced rapid-cycling affective disorder successfully treated with valproate. Psychosomatics 1999; 40:448-449Crossref, Medline, Google Scholar
20 Giakas WJ, Seibyl JP, Mazure CM: Valproate in the treatment of temper outbursts. J Clin Psychiatry 1990; 51:525Medline, Google Scholar
21 Horne M, Lindley SE: Divalproex sodium in the treatment of aggressive behavior and dysphoria in patients with organic brain syndromes. J Clin Psychiatry1995; 56:430-431Google Scholar
22 Sandel ME, Mysiw WJ: The agitated brain injured patient, part 1: definitions, differential diagnosis, and assessment. Arch Phys Med Rehabil 1996; 77:617-623Crossref, Medline, Google Scholar



