The Reliability and Clinical Correlates of Figure-Ground Perception in Schizophrenia
Abstract
Schizophrenia subjects are impaired in a number of visual attention paradigms. However, their performance on tests of figure-ground visual perception (FGP), which requires subjects to visually discriminate figures embedded in a rival background, is relatively unstudied. We examined FGP in 63 schizophrenia patients and 27 control subjects and found that the patients performed the FGP test reliably and had significantly lower FGP scores than the control subjects. Figure-ground visual perception was significantly correlated with other neuropsychological test scores and was inversely related to negative symptoms. It was unrelated to antipsychotic medication treatment. Figure-ground visual perception depends on “top down” processing of visual stimuli, and thus this data suggests that dysfunction in the higher-level pathways that modulate visual perceptual processes may also be related to a core defect in schizophrenia.
Figure-ground visual perception (FGP) tests require subjects to visually discriminate which figures are embedded in a rival background. Figure-ground visual perception performance generally improves with development in school age children, in line with the maturation of perceptual and visual memory systems.1 Normal adults show no ceiling effects on their ability to visually discriminate embedded figures and perform the test reliably. Scores in adult men are unrelated to education or socioeconomic status, although women's scores show a moderate association with education.2,3 Figure-ground visual perception tests are reported to be sensitive indicators of neural dysfunction and have been used to examine age related cognitive changes in older adults, cerebrovascular injury patients, and individuals with neurodegenerative disorders.4–7
The visual processes for figure-ground organization appear to depend on input from high-level cortical regions involved in object recognition, which orient spatial attention so that salient objects can be detected and separated from a background.8,9 The “top down” modulation of primary visual areas for spatial localization is accomplished through the ventral parvocellular pathway (“what is it”), which projects ventrally to the temporal lobe to encode specific details for object identification, and the dorsal magnocellular pathway (“where is it”), which is involved in detecting and segregating objects from the background. The magnocellular pathway projects to the parietal lobe to facilitate the orientation of attention and eye movements to salient stimuli for the initial detection of targets from the background.10
Figure-ground visual perception may also be a useful test for probing the neural underpinnings of schizophrenia, a disorder marked by significant neurocognitive impairments. Schizophrenia patients have intact visual perceptual ability and spatial orienting responses to external stimuli, but many schizophrenia patients are impaired in the higher-order processing of visual information. They are deficient in tasks that require internally generated control of selective spatial attention, including backwards masking, continuous performance tasks, and eye movement tasks that necessitate voluntary maintenance of gaze direction.11–18 These impairments implicate dysfunctional modulation of early visual processing by higher-order regions.19,20 Butler et al.21 recently showed that an abnormal magnocellular pathway, in particular explained the deficient “top down” modulation of early visual processing in schizophrenia patients.
There are only two contemporary studies concerning FGP performance in schizophrenia, and both conflicting reports concern FGP's relationship with symptomatology. Liddle22 reported that the FGP scores in schizophrenia patients were associated with positive symptoms of reality distortion, which include delusions and hallucinations. To the contrary, Malaspina et al.23 recently found a far more robust relationship of FGP with negative symptoms, particularly with poor rapport and lack of spontaneity.23 However, important characteristics of FGP test results in schizophrenia patients, such as the reliability of their FGP test performance and whether FGP scores are affected by antipsychotic medications, have not been previously described. It is likely that either poor reliability of FGP tests or significant medication effects could explain the discrepant results found within the literature.
To better understand the utility and application of FGP performance in schizophrenia research, we examined FGP in a sample of well-characterized schizophrenia patients, comparing their performance to that of healthy control subjects and examining effects of gender and education. We also assessed the reliability of FGP test results in schizophrenia patients and examined whether antipsychotic medications affected FGP performance. Finally, we examined the extent to which FGP ability was associated with performance on other neuropsychological measures in schizophrenia patients.
METHODS
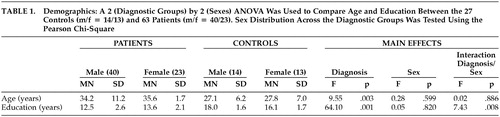
The patient sample included 63 patients with DSM-IV schizophrenia or other psychoses (40 men, 23 women) from a schizophrenia research unit. The DSM-IV diagnoses represented a consensus among clinical and research staff and considered information from structured interviews with the Diagnostic Interview for Genetic Studies,24 past and present hospital records, and information about the patients from family interviews and symptom ratings. The patients were typically admitted to the unit in stable clinical condition from either inpatient or outpatient treatment. They were physically healthy, with recent physical examinations, laboratory tests, urinalysis, and thyroid function tests, and all provided written informed consent for this institutional review board, approved study. The 27 healthy subjects evaluated for FGP (14 men, 13 women) were recruited from the medical center community. They had been clinically screened to exclude subjects who had any axis I disorder in the last 2 years or any personal or family history of psychosis.
Figure-Ground Perception
The Southern California Figure-Ground Visual Perception Test is a subtest of the Sensory Integration and Practice Tests25 and is divided into two parts, each containing eight stimuli. The first part, which we considered a control task, contains line drawings of easily identified familiar common pictures. The second part of the test contains abstract shapes embedded within the figure. Each background stimulus has an accompanying response card with six possible choices, three of which are contained within the stimulus, while three other figures serve as distracters. The instructions were read to the subjects, who then practiced on an unscored example before each part of the test. All subjects who regularly wore corrective eyewear were asked to do so during testing to insure intact visual acuity. They were asked to visually examine each stimulus and then select three of the six possible figures on the response card that was embedded in the background stimulus. They were told not to name or trace the shapes or pictures. If they chose incorrectly on the practice example, the instructions were repeated until they were able to choose the correct answers, but no feedback was given once the scored section began. The test scores were computed as the number of responses that were correct on the identification of common objects (picture control task) or the abstract geometric shapes (FGP). Since there were six possible choices on each response card and eight stimuli for each FGP subtest, the maximum score for either FGP subtest was 48.
We performed a total of 90 “first time” FGP evaluations on the combined sample of 63 patients and the 27 comparison subjects. Because we were interested in the stability of FGP test performance in the patients and any possible effects of medication and clinical state on the measure, we noted the treatment condition for all patient testing sessions and retested a portion of the 63 patients. The comparison subjects were tested only once. For the “first time” testing of patients, 47 were tested during stable antipsychotic treatment, and 16 were not receiving any antipsychotic medications. Thirty-two of the 63 patients were retested a month later. Twenty were retested on the same antipsychotic medication dose. Two were medication free on both test occasions, and 10 were tested during both antipsychotic treatment and medication-free conditions. No patient was medication-withdrawn for the purpose of this study.
Neuropsychological and Symptom Assessments
The neuropsychological test battery included the Wechsler Adult Intelligence Scale (performance, verbal, and full-scale IQ), Animal Naming, Trails B, and the Wisconsin Card Sorting Test (WCST). Scheduling demands of the patients and staff, lack of patient cooperation, and patient discharge reduced the sample of those who received neuropsychological testing from 63 to 39 patients. Patients' symptoms were assessed for the patients with the Positive and Negative Symptom Scale (PANSS),26 which was administered contemporaneously with the FGP testing. The PANSS data was quantified into the positive, negative, general psychopathology, and composite scales. The symptom data alone has been previously reported.23
Data Analysis
Sex distributions in the patient and control groups were examined with a Pearson chi-square test and the groups were compared on their age and education using analysis of variance. The patient group was significantly older and less educated than the normal comparison group, so age and education were included as covariates in comparing FGP performance between the patient and control groups. The analysis of covariance (ANCOVA) revealed a significant interaction of diagnosis and sex on FGP scores. Therefore, additional post hoc ANCOVAs were performed within sex, across the two groups, as well as within the groups and across sex. Paired t tests were performed to address test-retest performance of patients tested twice during the same active treatment phase and to assess medication effects in the patients tested during both antipsychotic treatment and after withdrawal from medication for at least 3 weeks. Correlations were performed between FGP scores and neuropsychological test results and with PANSS symptom measures. All probabilities were two-tailed and set at the 0.05 alpha level.
RESULTS
The gender composition did not differ between the patient group (40 men and 23 women) and the control group (14 men and three women) (χ2=1.07, df=1, p=0.30), although the patients were significantly older and less educated than the comparison subjects, and there was a significant interaction between group and sex for education. The men in the control group were better educated, whereas the women were better educated in the patient group (Table 1).
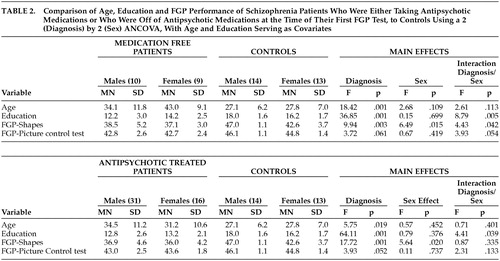
Table 2 separately presents the data for the first FGP test session based on patients' medication treatment status. Whether they were being treated with stable doses of antipsychotic medications or were not receiving any antipsychotic medications, patients had significantly lower scores on the FGP of abstract shapes test, but only marginally worse performance on the pictures task. Both male and female patients had lower scores than their control group counterparts, but there was a significant interaction of diagnosis and sex that was specifically related to the superior performance of the male comparison group subjects for both subtests. Post hoc ANCOVA analyses, conducted because the medication-free testing showed an interaction of diagnosis and sex, showed that the comparison group men had better performance than the comparison group women on the pictures test (F=6.27, df=1, 23, p=0.02) and the abstract shapes test (F=10.61, df=1, 23, p=0.003). However, there was no gender difference for the schizophrenia patients on either the pictures test (F=1.02, df=1, 15, p=0.328) or the abstract shapes test (F=0.16, df=1, 15, p=0.70).
The patient scores were reliable, and there was no improvement from practice. Those patients retested after 30 days on stable medication doses had unchanged scores for the embedded pictures (mean=42.5, SD=3.0, versus mean=42.9, SD=2.7) (t=1.19, df=18, p=0.249) and embedded abstract shapes FGP tests (mean=37.2, SD=4.9, versus mean=38.1, SD=5.3) (t=1.25, df=18, p=0.226). Accordingly, the patients showed strong test 1 to test 2 correlations for the pictures test (r=0.86, df=18, p=0.001) and the shapes test FGP (r=0.84, df=18, p=0.001).
Patients who were tested both on and off antipsychotic medications had scores that showed an absence of medication effects on FGP testing, since their scores were similar for the pictures test (mean=42.7, SD=1.4, versus mean=43.1, SD=2.1) (paired t=0.51, df=9, p=0.62) and the shapes test (mean=37.0, SD=3.5, versus mean=38.2, SD=4.0) (paired t=0.73, df=9, p=0.48). Medication treatment of patients with typical versus atypical antipsychotics was unrelated to the FGP scores. The particular schizophrenia-related diagnoses of the patients (21 schizoaffective disorder, 36 schizophrenia, six psychosis not otherwise specified or schizophreniform disorder) were unrelated to FGP performance scores.
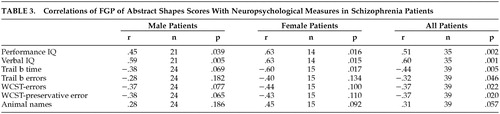
Among the 39 patients (70% of the men and 77% of the women) who had neuropsychological evaluations (Table 3), the men had a WAIS full-scale IQ of 82.6 (SD=12.8), and the women had a WAIS full-scale IQ of 83.6 (SD=12.0). The FGP pictures control test scores were unrelated to any of the neuropsychological test results, while the FGP abstract shapes scores were significantly positively correlated with full-scale, verbal, and performance IQ and were significantly negatively correlated with Trails B time and errors, WCST errors, and WCST perseverative responses. There was only a trend relationship between FGP of shapes and the animal naming verbal fluency test. As we have recently described,23 47 of these patients received PANSS clinical symptom ratings at the time of FGP testing. The FGP pictures control test scores were unrelated to any of the PANSS symptom subscales. The FGP abstract shapes scores were significantly related (across the sexes and ultimately for the whole patient group) with the negative symptom subscale (−0.47, df=47, p=0.001), but not with the positive symptom subscale (−0.19, df=47, p=0.20), the general psychopathology subscale (−0.26, df=47, p=0.08), or the composite scale (0.20, df=47, p=0.180).
DISCUSSION
We demonstrated that the ability of schizophrenia patients to perform the Southern California Figure-Ground Visual Perception Test could be reliably assessed and that their performance was unrelated to antipsychotic medication treatment or to a diagnosis of schizophrenia versus other psychoses such as schizoaffective disorder and unspecified functional psychosis. Schizophrenia patients showed significantly worse performance than control subjects on the abstract shapes section of the FGP test, a task that requires the visual segregation of embedded nonfigurative shapes as target figures from background stimuli. The patients had similar performance to the comparison subjects for the FGP control task that uses familiar identifiable pictures as the objects for the target stimuli. The deficient scores on the FGP abstract shapes task were not corrected by controlling for sex or educational achievement.
The capacity to distinguish which abstract shapes were embedded in the background was significantly associated with patients' intelligence and performance on several other neuropsychological tests and, as previously reported, FGP scores were inversely associated with PANSS negative symptom ratings. In contrast, the FGP scores for identifying the familiar pictures, which did not differ between the patient and control groups, were unrelated to neuropsychological test scores or symptoms.23 Spatial ability is considered to be a suitable index of general intelligence, and it is not surprising that it correlated with intelligence and the other neuropsychological measures in the patients. Additionally, negative symptoms in schizophrenia have been associated with impairments in other neuropsychological tests, including measures from the present battery such as Continuous Performance Test perseverative errors, Trails B time and errors, intelligence, and verbal fluency.27–31
These data showing that schizophrenia patients had FGP impairments when the targets were abstract shapes (“where is it”) and near normal performance when the targets were common namable objects (“what is it”) is arguably consistent with greater dysfunction, respectively, in the “top down” modulation by the magnocellular pathway than by the parvocellular pathways. “Top-down” information about a specific object (i.e., chair, bucket, cat) from the ventral parvocellular visual stream may facilitate the localization of the identifiable objects.32 Defective processing in magnocellular pathways may also be relevant to higher-level cognitive deficits. Other research has associated magnocellular deficits with predominantly negative symptoms,33 which would also be in line with the current data.
Other neural dysfunctions may also explain the FGP impairments in schizophrenia. Spatial ability has been associated with right hemisphere activity,34 and subgroups of schizophrenia patients are reported to have right hemisphere dysfunction and more treatment refractory negative symptoms.35,36 Additionally, FGP assesses topographical orientation and may be especially vulnerable to damage to the neurocircuitry connecting the primary sensory cortex to the inferior temporal cortex, hippocampus, and frontal cortex. The hippocampus has a special role in combining information to form spatial representations.37 If dysfunction in the prefrontal and temporoparietal cortices mediate the deficient sensory perception in schizophrenia, it may be because of abnormal “top-down” modulation.
Male control subjects scored significantly higher than female control subjects, but there was no sex difference in the schizophrenia patients. This sex difference in the healthy comparison group is consistent with other literature showing a male advantage in FGP tasks.38 The small mean advantage in scores for men on visual-spatial tasks may be a consequence of the greater proportion of higher-achieving men than of higher-achieving women,39 which this control data would support. The male advantage in FGP observed in some healthy male subjects may be offset in schizophrenia samples by the overall greater severity of illness typically seen in male schizophrenia patients.
A potential limitation of this study is that factors such as attention or the subjects' level of effort could have impacted the results. Although these problems can arise during behavioral testing, it is unlikely they could have accounted for the performance differences found between patients and control subjects on distinguishing abstract shapes in an embedded background due to the inclusion of the control picture task. All patients and control subjects were tested on the control task and experimental task under the same conditions, and accordingly, attention changes and level of effort were accounted for and regarded as the same for subjects across conditions. Additionally, to consider possible visual deficits such as acuity, we required subjects to wear their prescription lenses during testing.
These data suggest that FGP testing could be a worthwhile task for examining visual spatial processing in schizophrenia and may be a useful probe of the pathways that modulate visual perception. It is easily administered and brief, typically taking less than 30 minutes for the patients to complete and can assess visuospatial discrimination without the significant confounding from memory or motor demands that other visuospatial tasks entail. The neural deficits that underlie negative symptoms and neurocognitive functioning in schizophrenia may share common substrates with the distributed neural network necessary for FGP, particularly that which subserves the voluntary visuospatial processing and spatial representation.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Xavier Amador and Alan Brown and Nora Goudsmit, M.A, for their contributions to this study. This work was supported by the Mathers Foundation and by NIMH grant 5P20 MH-50727.
 |
 |
 |
1 Rohr ME, Ayers JB: Problems encountered in development of instrumentation to measure figure-ground perception among young children. Percept Mot Skills 1976; 43:1221–1222Crossref, Medline, Google Scholar
2 Petersen P, Wikoff RL: The performance of adult males on the Southern California Figure-Ground Visual Perception Test. Am J Occup Ther 1983; 37:554–560Crossref, Medline, Google Scholar
3 Petersen P, Goar D, Van Deusen J: Performance of female adults on the Southern California Visual Figure-Ground Perception Test. Am J Occup Ther 1985; 39:525–530Crossref, Medline, Google Scholar
4 Roper BL, Bieliauskas LA, Basso MR, et al: The influence of age and impairment status on the Southern California Figure-Ground Visual Perception Test (abstract). J Int Neuropsychol Soc 1995; 1:169Google Scholar
5 Roper BL, Fiengo J, Holker EG, et al: Older adult norms for the Southern California Figure-Ground Visual Perception Test. Clin Neuropsychol 2001; 15:324–328Crossref, Medline, Google Scholar
6 Egelko S, Gordon WA, Hibbard MR, et al: Relationship among CT scans, neurological exam, and neuropsychological test performance in right-brain-damaged stroke patients. J Clin Exp Neuropsychol 1988; 10:539–564Crossref, Medline, Google Scholar
7 Bieliauskas LA, Roper BL, Trobe J, et al: Cognitive measures, driving safety, and Alzheimer's disease. Clin Neuropsychol 1998; 12:206–212Crossref, Google Scholar
8 Vecera SP, O'Reilly RC: Figure-ground organization and object recognition processes: an interactive account. J Exp Psychol Hum Percept Perform 1998; 24:441–462Crossref, Medline, Google Scholar
9 Peterson MA, Gibson BS: Object recognition contributions to figure-ground organization: operations on outlines and subjective contours. Percept Psychophys 1994; 56:551–564Crossref, Medline, Google Scholar
10 Merigan WH, Maunsell JHR: How parallel are the primate visual pathways? Annu Rev Neurosci 1993; 16:369–402Crossref, Medline, Google Scholar
11 Sereno AB, Holzman PS: Spatial selective attention in schizophrenic, affective disorder, and normal subjects. Schizophr Res 1996; 20:33–50Crossref, Medline, Google Scholar
12 O'Donnell BF, Swearer JM, Smith LT, et al: Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry 1996; 153:687–692Crossref, Medline, Google Scholar
13 Fleming K, Goldberg TE, Binks S, et al: Visuospatial working memory in patients with schizophrenia. Biol Psychiatry 1997; 41:43–49Crossref, Medline, Google Scholar
14 Green MF, Nuechterlein KH, Mintz J: Backward masking in schizophrenia and mania, II: specifying the visual channels. Arch Gen Psychiatry 1994; 51:945–951Crossref, Medline, Google Scholar
15 Saccuzzo DS, Cadenhead KS, Braff DL: Backward versus forward visual masking deficits in schizophrenic patients: centrally, not peripherally, mediated? Am J Psychiatry 1996; 153:1564–1570Crossref, Medline, Google Scholar
16 Kelley MP, Bakan P: Eye tracking in normals: spem asymmetries and association with schizotypy. Int J Neurosci 1999; 98:27–81Crossref, Medline, Google Scholar
17 Nkam I, Thibaut F, Denise P, et al: Saccadic and smooth-pursuit eye movements in deficit and non-deficit schizophrenia. Schizophr Res 2001; 48:145–153Crossref, Medline, Google Scholar
18 Cornblatt BA, Keilp JG: Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull 1994; 20:31–46Crossref, Medline, Google Scholar
19 Adler LE, Freedman R, Ross RG, et al: Elementary phenotypes in the neurobiological and genetic study of schizophrenia. Biol Psychiatry 1999; 46:8–18Crossref, Medline, Google Scholar
20 Braff DL, Saccuzzo DP, Geyer MA: Information processing dysfunctions in schizophrenia: studies of visual backward masking, sensorimotor gating, and habituation, in Handbook of Schizophrenia. Edited by Steinhauer SR, Gruzelier JH, Zubin J. New York, Elsevier, 1991, pp 303–334Google Scholar
21 Butler PD, Schechter I, Zemon V, et al: Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry 2001; 158:1126–1133Crossref, Medline, Google Scholar
22 Liddle PF: Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol Med 1987; 17:49–57Crossref, Medline, Google Scholar
23 Malaspina D, Simon N, Corcoran C, et al: Using figure ground perception to examine the unitary and heterogeneity models for psychopathology in schizophrenia. Schizophr Res 2003; 59:297–299Crossref, Medline, Google Scholar
24 Nurnberger JI Jr, Blehar MC, Kaufmann CA, et al: Diagnostic Interview for Genetic Studies: rationale, unique features, and training. Arch Gen Psychiatry 1994; 51:849–859Crossref, Medline, Google Scholar
25 Ayres AJ: Southern California Figure-Ground Visual Perception Test Manual. Los Angeles, Western Psychological Services, 1966Google Scholar
26 Kay SR, Opler LA, Lindenmayer JP: The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl 1989; 7:59–67Medline, Google Scholar
27 Perry W, Braff DL: A multimethod approach to assessing perseverations in schizophrenia patients. Schizophr Res 1998; 33:69–77Crossref, Medline, Google Scholar
28 Rossi A, Daneluzzo E, Mattei P, et al: Wisconsin Card Sorting Test and Stroop test performance in schizophrenia: a shared construct. Neurosci Lett 1997; 226:87–90Crossref, Medline, Google Scholar
29 Voruganti LN, Heslegrave RJ, Awad AG: Neurocognitive correlates of positive and negative syndromes in schizophrenia. Can J Psychiatry 1997; 42:1066–1071Crossref, Medline, Google Scholar
30 Berman I, Viegner B, Merson A, et al: Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophr Res 1997; 25:1–10Crossref, Medline, Google Scholar
31 Mattson DT, Berk M, Lucas MD: A neuropsychological study of prefrontal lobe function in the positive and negative subtypes of schizophrenia. J Genet Psychol 1997; 158:487–494Crossref, Medline, Google Scholar
32 Moore CJ, Price CJ: A functional neuroimaging study of the variables that generate category-specific object processing differences. Brain 1999; 122:943–962Crossref, Medline, Google Scholar
33 Slaghuis WL, Bishop AM: Luminance flicker sensitivity in positive- and negative-symptom schizophrenia. Exp Brain Res 2001; 138:88–99Crossref, Medline, Google Scholar
34 Ardila A: Historical evolution of spatial abilities. Behav Neurol 1993; 6:83–87Crossref, Medline, Google Scholar
35 Malaspina D, Harkavy-Friedman J, Kaufmann C, et al: Psychobiological heterogeneity of familial and sporadic schizophrenia. Biol Psychiatry 1998; 43:489–496Crossref, Medline, Google Scholar
36 Malaspina D, Goetz RR, Harkavy Friedman J, et al: Traumatic brain injury and schizophrenia in members of schizophrenia and bipolar disorder pedigrees. Am J Psychiatry 2001; 158:440–446Crossref, Medline, Google Scholar
37 Kolb B, Whishaw IQ: Fundamentals of Human Neuropsychology. New York, WH Freeman, 1990Google Scholar
38 Pratarelli ME, Steitz BJ: Effects of gender on perception of spatial illusions. Percept Mot Skills 1995; 80:625–626Crossref, Medline, Google Scholar
39 Hedges LV, Nowell A: Sex differences in mental test scores, variability, and numbers of high-scoring individuals. Science 1995; 269:41–45Crossref, Medline, Google Scholar



