Relationships Between Cognitive and Neurological Performance in Neuroleptic-Naïve Psychosis
Abstract
The authors explored relationships between neuropsychological performance and neurological exam abnormalities in 86 never-medicated patients with nonorganic psychosis (59 with schizophrenia or schizoaffective disorder) and 51 healthy subjects. Assessments include a reliable subset of the Neurological Evaluation Scale (rNES) and several neuropsychological tests of attention, executive function, memory, and current and premorbid intelligence. Principal components analysis of the rNES yielded two main factors. Of these, CogPer (consisting of more cognitively demanding perceptual tasks) showed stronger relationships than RepMot (consisting of repetitive manual motor tasks) to neuropsychological measures. Customarily, frontal neuropsychological tasks also relate more strongly to CogPer than to RepMot. Approximately one-half of the variability in these cognitive and neurological assessments is shared.
Neurologic and neuropsychological examinations have a mutual objective (assessing nervous system function) and approach (observing behavioral responses to specific stimuli and performance on specific tasks). Indeed, some neuropsychological batteries, such as the Luria-Nebraska,1 have many “sensorimotor” items in common with the neurological examination. However, these two approaches diverge in several ways that impede their integration. The neurological exam, representing over a century of clinical lore with partial empirical support, consists of items selected for their capacity to support or refute specific diagnoses or lesion locations. The examination tends to be briefer, with more variation in content and method from one patient to the next. Its results are usually scored in nominal or ordinal formats. Neuropsychological assessment, growing out of a more empirical psychometric tradition, consists of items selected to represent domains of performance. The examination is more lengthy, less flexible in content and more regimented in its method of administration. Its results are scored as continuous variables, which are transformed for ready comparison to the general population. These artificial distinctions interfere with a synthesis of two inherently similar types of assessment. For both research and clinical applications, efficiency requires that we determine the extent of redundancy between these two types of assessment.
Several studies have compared the broad outcomes of neurological and neuropsychological evaluations in neuropsychiatric patients, consistently finding positive relationships.2,3 Jenkyn et al.4 specifically found that performance on a brief neurological exam predicted global neuropsychological impairment in a heterogeneous patient group. However, some of these studies compare batteries with overlapping content (neurological exams including cognitive tasks, and vice versa), and they use summary indices that leave open the possibility that certain domains of the two procedures are distinct.
In schizophrenia, bedside neurological examinations have generally demonstrated nonspecific neurological impairment.5,6 Many possible confounds such as exposure to neuroleptic medications, socioeconomic factors, and chronic illness have been successfully discounted in studies of first-episode, never-treated schizophrenia, that have consistently found greater global impairment in schizophrenic than healthy control groups.7–9 This conclusion parallels the neuropsychological literature, in which first-episode schizophrenic patients show generalized impairment.10,11 However, we know little about the meaning of neurological impairment in these patients, and the possibility remains that such factors as effort, attention to the tasks, or understanding and recall of directions are responsible.5,6 Such factors impact on neurological and neuropsychological performance alike, so that the nature and extent of shared variance may be informative as to the source of neurological impairment in schizophrenia. If neurological and neuropsychological impairment are strongly and consistently related throughout both data sets, this would detract from the validity of the neurological “soft” signs and would suggest that they are referable to cognitive or motivational limitations. If the shared variance is limited or isolated to specific domains of neurological or cognitive performance, this would support the potential of the neurological examination to provide independent and valid information.
Several studies have compared neurological examination abnormalities (NEA) and neuropsychological performance in schizophrenia. Most studies of chronic schizophrenia have found that global NEA indices are related to global neuropsychological indices.12–16 In a study of treated inpatients with schizophrenia,14 global intelligence and several putatively frontal tasks related to performance on the Neurological Evaluation Scale (NES) and each of its subscales to a similar extent. Another group,17 using data in which most of the variability occurred on motor tasks, found global NEA related only to predominantly motor tasks from their neuropsychological battery. A study of the NES and its original subscales18 in a chronic group found that the summary score, motor sequencing (Luria frontal motor tasks) and motor coordination (cerebellar tasks) related to global cognition, but that the sensory integration scale was independent of cognition.19 In another chronic and medicated population, Arango et al.20 found essentially the opposite: that sensory integration more often predicted neuropsychological variables. Wong et al.15 compared subscales of a neurological battery in terms of relationships with various neuropsychological measures and found that a perceptual score was more closely related than a motor score to 9 of 10 neuropsychological variables. Results to date indicate that NEA and neuropsychological performance are related in schizophrenia but do not clarify whether or how specific domains of NEA or of neuropsychological performance are related.
One source of inconsistency may be the presence of antipsychotic medication, which may distort relationships between variables. Expected relationships between neurocognitive performance and negative symptoms have often been found only among patients taking antipsychotic drugs.21–23 Another difficulty in comparing studies is the inconsistent composition of scales and subscales.6 Proposed NEA subscales have been based on factor analyses24–26 and on neuroanatomic considerations.7,18,27,28 We believe that statistically derived NEA factors offer greater power than do individual neurological abnormalities, with more empirically defensible validity than the anatomically based subscales. Our factor analysis of unmedicated patients with schizophrenia26 yielded four factors. One of these, the “cognitive-perceptual” (CogPer) factor, consisting of more cognitively demanding tasks, was associated with estimated IQ. We predicted on this basis that CogPer would be more strongly associated with cognitive performance than the other factors.
To contribute to the integration of the neurological and neuropsychological examinations, and to better understand the significance of NEA in schizophrenia, we examined the relationships between neuropsychological and neurological performance in first-episode, neuroleptic-naïve schizophrenia and in normal comparison subjects. Such a sample is free of the possibly confounding effects of past or present medication on neurological or neuropsychological function, and lays the groundwork for future studies evaluating the effects of medication on these functions. We sought to cross-validate the previously reported factor structure of the NES,26 to test the hypothesis that CogPer is selectively associated with cognitive performance, and to determine the nature and extent of shared variance between cognitive and neurological performance in psychosis.
METHOD
Subjects
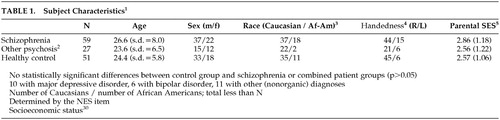
Subjects included individuals who were involved in a longitudinal study of first-episode psychosis.29 Some of the current sample were part of our previous factor analysis.26 Eighty-six patients with never-treated psychosis were evaluated with thorough medical, neurological, and psychiatric evaluations. Of these subjects, 59 were diagnosed with schizophrenia or schizoaffective disorder, and 27 were diagnosed with other psychotic disorders. Patients were recruited from the inpatient and outpatient services of the Western Psychiatric Institute and Clinic, Pittsburgh. All subjects were interviewed using the Structured Clinical Interview for DSM-IV. Diagnoses were derived by consensus diagnostic evaluations and were formally confirmed after at least 6 months’ follow-up.
First episode psychotic patients were ages 15 to 45, met DSM-IV criteria for a nonorganic psychotic disorder, and had no prior treatment with antipsychotic drugs. We excluded patients with significant medical or neurological illness, mental retardation as defined by IQ <75, head injury with loss of consciousness temporally related to psychosis onset, current substance abuse, or substance dependence within the previous six months.
Healthy subjects were recruited by advertisement in local neighborhoods and communities in which the patients reside. These subjects had no current or past Axis I disorder, neurological disorders, or other chronic medical conditions, IQ <75, or history of schizophrenia or major mood disorder in first-degree relatives. None had been exposed to any psychotropic medication within 6 months of baseline assessment. This group did not significantly differ from the patient group in age, gender, race, or parental socioeconomic status.30
Neurological Evaluation
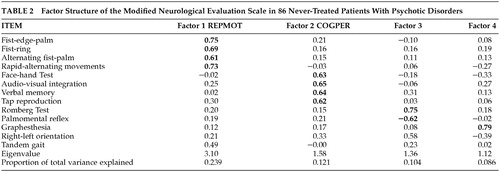
Neurological evaluations were carried out using a modified version of the NES18 administered as part of the baseline evaluation, prior to beginning antipsychotic medication. The original 29-item NES was supplemented with the palmomental reflex,31 and then reduced to 13 items (Table 2). This reduction involved unifying scores from bilaterally administered items by including the higher of the two ratings, and by omitting lateral dominance items, items in which <10% of subjects had abnormalities, and items which were not consistently reliably rated.32 Those conducting these assessments have established inter-rater reliability as described previously,32 and have continued to do NES assessments together to avoid rater drift. This 13-item revised NES (rNES) was used for all data analyses in this study.
Neuropsychological Assessment
The battery used included 11 cognitive neuropsychological tasks. General intelligence was estimated using the Ammons Quick Test,33 and premorbid intelligence was estimated using the vocabulary subtest of the Wide Range Achievement Test (WRAT).34 Verbal learning and memory were assessed with the California Verbal Learning Test25 total correct for the five list A learning trials. Verbal fluency was assessed with the Controlled Word Association Test.36 Attention was assessed using the Digit Span and Digit Symbol tests from the Wechsler Adult Intelligence Scale—Revised (WAIS-R)37 and Trail Making Form B.38 Wisconsin Card Sorting Test39 perseverative errors assessed executive dysfunction. Visual perception and memory were assessed with the Benton Judgment of Line Orientation Test40 and with the immediate and delayed visual memory design reproduction of the Wechsler Memory Scale—Revised (WMS-R).41 We did not include neuropsychological tests of basic sensory and motor abilities because of their resemblance to neurological exam procedures.
Treatment of Data
The 13 rNES items were entered into a Principal Components Analysis with Varimax rotation. Factor scores were computed by adding the scores with salient loadings on each of the factors. Neuropsychological test scores were transformed to standard “T” scores based on the total sample, to allow for placing all scores on the same scale. The mean of these standard scores for each subject was utilized as a general index of impairment.
Data were then analyzed in several ways. First, we computed Pearson r correlations between total rNES scores and the two dominant rNES factor scores and neuropsychological test scores. Second, we used multiple regression, with the two factor scores as independent measures and neuropsychological test scores as dependent measures. All dependent variables were entered initially, followed by stepwise analyses. We also performed a multiple regression analysis in the reverse direction using the mean neuropsychological standard score as the independent measure and the rNES items as the dependent measures. Finally, we used canonical correlation, with the 13 rNES items as the first set and the 11 neuropsychological tests as the second, to derive an overall index of the strength of association between the rNES and the neuropsychological test battery.
RESULTS
Demographic data are presented in Table 1. There were no statistically significant (P<0.05) differences among the schizophrenia, other psychosis, and control groups on any of the demographic variables examined. For individual rNES items, the frequency of abnormal findings (score of 1 or 2) among patients ranged from 3% to 63%; the frequency of markedly abnormal findings (score of 2) varied from 0% to 40%. The corresponding frequencies among healthy control subjects were 0% to 47% (score of 1 or 2) and 0% to 20% (score of 2). The mean score for the 13 rNES items in the schizophrenia group was 0.64, in the other psychosis group 0.59, and among healthy control subjects 0.25. These scores are comparable to those in other studies of schizophrenia using the NES.14,18 For the rNES total score, a one way analysis of variance revealed a significant difference among the three groups (F=22.07, df=2, 130, p<0.001). A Scheffé test indicated that the schizophrenia and other psychosis group did not differ from each other, both differing from the healthy group (p<0.05). The mean standard T score for the cognitive tests was also significantly different among groups (F=17.02, df=2, 112, p<0.001). The Scheffé test again indicated that the schizophrenia and other psychosis groups did not differ from each other, and that both differed from the healthy control group (p<0.05). Variances were also similar across groups for both cognitive and neurological summary scores. These results led us to combine the schizophrenia and other psychosis groups for purposes of the factor analysis and regression analyses.
Principal components factor analysis of the rNES in the patient sample is presented in Table 2. Compared with our previous result,26 RepMot is unchanged (fist-ring, fist-edge-palm, alternating fist-palm, diadochokinesis). CogPer loses one item (right-left orientation), leaving audiovisual integration, verbal memory, tap-copying and face-hand test. The remaining two factors are substantially altered. For subsequent analyses, we employed RepMot and CogPer only, each computed by summing the scores of their four component tests.
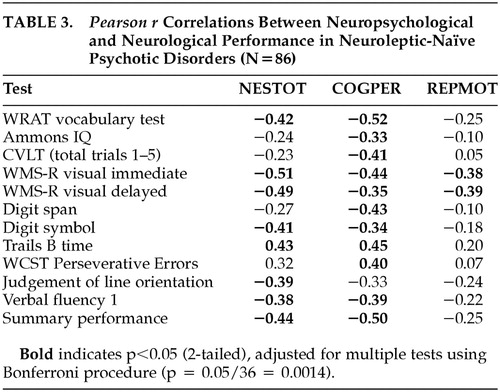
Pearson r correlations between rNES total score, RepMot and CogPer scores and each cognitive neuropsychological variable, are presented in Table 3. As demonstrated in this table, correlations are consistently in the expected direction, and range from weak to moderately strong. Because of the large number of correlations (comprising a 3-by-12 grid of values), p<0.0014 (0.05 divided by 36) was used as the probability level for determining whether correlations were significantly different from zero. Seven of the eleven cognitive measures correlated significantly with overall NES performance. The correlation between total rNES and the mean neuropsychological test standard score was r=−0.44 (p<0.001). CogPer correlated significantly with 10 of 11 individual neuropsychological tests, and with the mean standard score (r=−0.50, p<0.001). RepMot showed a weaker association with the standard score (r=−0.25, NS), reaching significance only with the Wechsler visual memory tests. No correlations reached statistical significance in the healthy control group. Subsequent analyses were limited to the patient group.
To assess cognitive associations with rNES performance, multiple regression analyses included the eleven cognitive tests as independent variables, and RepMot and CogPer in turn as dependent variables. For the RepMot factor, the multiple correlation coefficient obtained when all variables were entered was R=0.55, explaining 29.7% of the variance. When the stepwise method was used, R=0.45, explaining 19.9% of the variance. Using default options, the variables entered were the WAIS Digit Symbol test (t=−3.19, p<0.005) and WMS-R visual memory—immediate recall (t=−2.46, p<0.05), both of which involve visual processing and memory. For the CogPer factor, when all variables were entered, R was equal to 0.69, explaining 46.9% of the variance. The stepwise analysis resulted in an R of 0.65, explaining 42.8% of the variance. Variables entered were Wisconsin Card Sorting Test perseverative errors (t=4.14, p<0.001), the WRAT reading subtest (t=−3.68, p<0.001), and the WMS-R visual memory—immediate recall (t=−2.73, p<0.01). Thus, CogPer is independently influenced by conceptual reasoning and premorbid intellectual performance, and both CogPer and RepMot are related to visual memory.
To assess the rNES as a potential predictor of neuropsychological performance, we entered the 13 individual rNES items into another multiple regression, with the mean standard neuropsychological test score as the dependent variable. With all variables entered, the multiple correlation coefficient was R=0.66, with 42.9% of the variance explained. Stepwise analysis resulted in an R of 0.59, explaining 34.9% of the variance. Tap-copying (t=−3.90, p<0.001), graphesthesia (t=−2.91, p<0.005) and tandem gait (t=−2.17, p<0.05) entered into the equation. We did an initial regression analysis to evaluate the association between the rNES factors and the mean neuropsychological test standard score. Entering RepMot, CogPer and the sum of scores for the items that did not load onto either RepMot or CogPer (these being Romberg, palmomental reflex, graphesthesia, right-left orientation, and tandem gait) into a stepwise regression, only CogPer entered (t=−3.43, p<0.001).
Canonical correlation included the 11 neuropsychological and 13 neurological variables. The general canonical R was 0.735, explaining 54% of the variance. A chi-square test for significance of the difference from zero was equal to 215.9 (p<0.0001).
DISCUSSION
We contrasted results on neurological and neuropsychological test batteries in never-medicated patients with idiopathic (“nonorganic”) psychotic disorders. As expected, we found that one neurological factor (CogPer) accounts for most of the relationship between rNES and cognitive performance. We found that visual memory covaries with neurological performance generally, and that measures of executive and premorbid intellectual functioning covary with performance in the perceptually oriented CogPer subscale of the rNES. Finally, we found that the rNES is moderately strongly related to neuropsychological performance, that CogPer and several individual neurological signs are associated with overall cognitive impairment.
The divergence in relationships between the rNES sub-scales and neuropsychological performance supports the neurological examination as a distinctive component in the assessment of the schizophrenic patient. It also supports the construct validity of our factor analyses, substantiating our previous interpretation of the second factor as “cognitive-perceptual.”26 This finding is consistent with an older body of research, in which general cognitive ability was found to be associated with performance in audiovisual integration and the face-hand test.42 The current factor analysis cannot be seen as replicating the results of our prior factor analysis, though, because the current sample overlaps with that used in our earlier study. The pattern of NEA relationships, with CogPer being related more strongly with traditionally frontal neuropsychological tasks contradicts anatomically based schemes for organizing NEA and neuropsychological performance in schizophrenia. For example CogPer was more strongly related than RepMot to such “frontal” tasks as verbal fluency and the Wisconsin Card Sorting Test. Rather than supporting an anatomically based scheme for organizing NEA, these results suggest that NEA may aggregate in terms of their dependence on cognitive functioning.
CogPer overlaps with the conceptually derived subscale “Sensory Integration,”18 which another group found to be more strongly related than motor subscales to neuropsychological performance in chronic, medicated schizophrenia,20 and which was found associated with the deficit syndrome.43 We have found that CogPer distinguishes schizophrenia-schizoaffective disorder from other idiopathic psychoses before treatment44 and predicts persistent negative symptoms after 2–4 years of treatment.45 Impaired performance on these perceptual tasks may be indicative both of the diagnosis of schizophrenia, and of chronic negative symptoms, which may be specific to schizophrenia.46
Our results, although consistent with those of some other studies,15,20 contrast with those of some others. Flashman et al.17 found that the shared variance between neurological and neuropsychological batteries was limited to the motor items in each group. This result likely reflects the low prevalence in nonmotor NEA in that study. A thorough study of the NES in clinically improved, medicated patients with schizophrenia14 found that motor sequencing related more strongly than sensory integration to executive functioning tests. Even after statistical correction for neuroleptic dose, Braun et al.47 found that motor NEA were as strongly related to a variety of cognitive tests as were perceptual NEA. In another study,19 significant relationships between NEA and cognition were limited to the motor sequencing (Luria frontal motor tasks) and motor coordination (cerebellar tasks) subscales, but details have not been published. These studies vary in subject characteristics and in the specific neurological assessment schedule used. None of the above studies were of neuroleptic-naïve patients, and none have used the specific subscales presented here. These differences may account for the inconsistent results among these studies.
Although we found significant relationships between these sets of data, the extent of these relationships is moderate, indicative of shared variance in the range of 40% to 50%. Our results suggest that a very brief examination, including tandem gait, graphesthesia and tap reproduction, could account for about 35% of the variance in overall cognitive performance. Stronger relationships might emerge in a more heterogeneous sample including subjects with secondary psychoses.
We found that performance on visual memory (WMS-R visual immediate memory) and speed of information processing (WAIS Digit Symbol) was associated with performance on the rNES. Visual processing is clearly involved in learning and performing motor sequencing tasks and certain other NEA (such as audiovisual integration). We might expect relationships between visual processing and RepMot to weaken with repeated examinations, when learning becomes less important a determinant of performance.
The relatively strong relationship between CogPer and the WRAT suggests that premorbid cognitive function may be more strongly related to these NEA than current cognition. British and Indian groups have found relationships between educational attainment, another proxy for premorbid intelligence, and NEA.48 The lack of a correlation between years of education and neurological performance in the current study may be attributable to differences between educational systems, such as differences in variability in years of education or in the extent to which years of education reflect academic performance. If NEA were in fact more strongly related to premorbid than to current intellectual performance, this would be consistent with the intuitive but unproven idea that neurological functioning is less variable over time than cognitive functioning. If NEA are relatively resistant to state influences, the neurological examination may hold particular potential as a prognostic aid.
Future studies will address the possibility that our results are specific to early psychosis or to the medication-naïve state, by reexamining these relationships in the same group over two years of follow-up. Similar studies using expanded batteries are required to more fully understand the extent of overlap between neurological and cognitive performance. Follow-up studies, based on presently emerging high-risk protocols, may permit prospective study of the links between NEA and premorbid versus morbid cognitive functioning.
Tests of CNS integrity can be seen as falling along a continuum based on task complexity. Neurological examinations tend to tap the lower end, and neuropsychological tests the higher end, of this spectrum. Although neurological and neuropsychological performance have shared variance in schizophrenia, this is primarily restricted to perceptual tasks.
ACKNOWLEDGMENTS
The authors thank Nancy McLaughlin and Joseph Pierri for conducting neurological assessments, and to Mary Kelly for statistical advice.
This work was supported in part by the Veterans Administration Medical Research Service (Drs. Sanders and Goldstein), the Novartis Foundation and the Swiss Foundation for Medical-Biological Grants (Dr. Schuepbach), NIMH grants MH-45203, MH-01180, and MH-45156 (Dr. Keshavan), and GCRC grant M01 RR-00056.
Presented at the International Congress of Schizophrenia Research, Wheeler, B.C., Canada, April 29–May 2, 2001; the American Neuropsychiatric Association, La Jolla, Calif., March 10–12, 2002.
 |
 |
 |
1 Golden CJ, Purisch A, Hammeke T: Luria-Nebraska Neuropsychological Battery Manual—Forms I and II. Los Angeles, Western Psychological Services, 1985Google Scholar
2 Quitkin F, Rifkin A, Klein DF: Neurologic soft signs in schizophrenia and character disorders. Arch Gen Psychiatry 1976; 33:845–853Crossref, Medline, Google Scholar
3 Stern Y, Marder K, Bell K, et al: Multidisciplinary baseline assessment of homosexual men with and without human immunodeficiency virus, II: neurologic and neuropsychological findings. Arch Gen Psychiatry 1991; 48:131–138Crossref, Medline, Google Scholar
4 Jenkyn LR, Walsh DB, Culver CM, et al: Clinical signs in diffuse cerebral dysfunction. J Neurol Neurosurg Psychiatry 1977; 40:956–966Crossref, Medline, Google Scholar
5 Heinrichs DW, Buchanan RW: Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry 1988; 145:11–18Crossref, Medline, Google Scholar
6 Sanders RD, Keshavan MS: The neurologic examination in adult psychiatry: from soft signs to hard science. J Neuropsychiatry Clin Neurosci 1998; 10:395–404Link, Google Scholar
7 Rubin P, Vorstrup S, Hemmingsen R: Neurological abnormalities in patients with schizophrenia or schizophreniform disorder at first admission to hospital: correlations with computerized tomography and regional cerebral blood flow findings. Acta Psychiatr Scand 1994; 90:385–390Crossref, Medline, Google Scholar
8 Sanders RD, Keshavan MS, Schooler NR: Neurologic exam abnormalities in first-break, neuroleptic-naive schizophrenia: preliminary results. Am J Psychiatry 1994; 151:1231–1233Crossref, Medline, Google Scholar
9 Gupta S, Andreasen NC, Arndt S, et al: Neurological soft signs in neuroleptic-naive and neuroleptic-treated schizophrenic patients and in normal comparison subjects. Am J Psychiatry 1995; 152:191–196Crossref, Medline, Google Scholar
10 Saykin AJ, Shtasel DL, Gur RE, et al: Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry 1994; 51:124–131Crossref, Medline, Google Scholar
11 Albus M, Hubmann W, Wahlheim C, et al: Contrasts in neuropsychological test profile between patients with first-episode schizophrenia and first-episode affective disorders. Acta Psychiatr Scand 1996; 94:87–93Crossref, Medline, Google Scholar
12 Owens DGC, Johnstone GC: The disabilities of chronic schizophrenia-their nature and the factors contributing to their development. Br J Psychiatry 1980; 136:384–395Crossref, Medline, Google Scholar
13 Liddle PF, Haque S, Morris DL, et al: Dyspraxia and agnosia in schizophrenia. Behav Neurol 1993; 6:49–54Crossref, Medline, Google Scholar
14 Mohr F, Hubmann W, Cohen R, et al: Neurological soft signs in schizophrenia: assessment and correlates. Eur Arch Clin Neurosci 1996; 246:240–249Crossref, Medline, Google Scholar
15 Wong AHC, Voruganti LNP, Heslegrave RJ, et al: Neurocognitive deficits and neurological signs in schizophrenia. Schizophr Res 1997; 23:139–146Crossref, Medline, Google Scholar
16 Merriam AE, Kay SR, Opler LA, et al: Neurological signs and the positive-negative dimension of schizophrenia. Biol Psychiatry 1990; 28:181–192Crossref, Medline, Google Scholar
17 Flashman LA, Flaum M, Gupta S, et al: Soft signs and neuropsychological performance in schizophrenia. Am J Psychiatry 1996; 153:526–532Crossref, Medline, Google Scholar
18 Buchanan RW, Heinrichs DW: The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res 1989; 27:335–350Crossref, Medline, Google Scholar
19 Mehrotra R, Soni W, Drodz S, et al: Neurological soft signs in chronic schizophrenia: clinical, cognitive, and structural correlates. Schizophr Res 2000; 45:262Crossref, Google Scholar
20 Arango C, Bartko JJ, Gold JM, et al: Prediction of neuropsychological performance by neurological signs in schizophrenia. Am J Psychiatry 1999; 156:1349–1357Medline, Google Scholar
21 Himmelhoch S, Taylor SF, Goldman RS, et al: Frontal lobe tasks, antipsychotic medication, and schizophrenia syndromes. Biol Psychiatry 1996; 39:227–229Crossref, Medline, Google Scholar
22 Condray R, van Kammen DP, Steinhauer SR, et al: Language comprehension in schizophrenia: trait or state indicator? Biol Psychiatry 1995; 38:287–296Crossref, Medline, Google Scholar
23 Allen DN, Anastasiou A, Goldstein G, et al: Influence of haloperidol on the relationship of frontal lobe function to psychomotor poverty and disorganization syndromes. Psychiatry Res 2000; 93:33–39Crossref, Medline, Google Scholar
24 Malla AK, Norman RMG, Aguilar O, et al: Relationship between neurological soft signs and syndromes of schizophrenia. Acta Psychiatr Scand 1997; 96:274–280Crossref, Medline, Google Scholar
25 Krebs M-O, Gut-Fayand A, Bourdel M-C, et al: Validation and factorial structure of a standardized neurological examination assessing neurological soft signs in schizophrenia. Schizophr Res 2000; 45:245–260Crossref, Medline, Google Scholar
26 Sanders RD, Keshavan MS, Forman SD, et al: Factor structure of neurologic examination abnormalities in unmedicated schizophrenia. Psychiatry Res 2000; 95:237–243Crossref, Medline, Google Scholar
27 Chen EYH, Shapleske J, Luque R, et al: The Cambridge Neurological Inventory: a clinical instrument for assessment of soft neurological signs in psychiatric patients. Psychiatry Res 1995; 56:183–204Crossref, Medline, Google Scholar
28 Egan MF, Hyde TM, Bonomo JB, et al: Relative risk of neurological signs in siblings of patients with schizophrenia. Am J Psychiatry 2001; 158:1827–1834Crossref, Medline, Google Scholar
29 Keshavan MS, Schooler NR, Sweeney JA, et al: Research and treatment strategies in first-episode psychoses: the Pittsburgh experience. Br J Psychiatry Suppl 1998; 172:60–65Crossref, Medline, Google Scholar
30 Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
31 Keshavan MS, Yeragani VK, Channabasavanna SM: A critical evaluation of infantile reflexes in neuropsychiatric diagnosis. Indian J Psychiatry 1979; 21:267–270Google Scholar
32 Sanders RD, Forman SD, Pierri JN, et al: Inter-rater reliability of the neurological examination in schizophrenia. Schizophr Res 1998; 29:287–292Crossref, Medline, Google Scholar
33 Ammons RB, Ammons CH: The Quick Test (QT): provisional manual. Psychol Rep 1962; 11:111–161Crossref, Google Scholar
34 Jastak S, Wilkinson GS: The Wide Range Achievement Test, Revised. Wilmington, Del, Jastak Associates, 1984Google Scholar
35 Delis DC, Kramer JH, Kaplan E, et al: California Verbal Learning Test, research ed. Cleveland, Psychological Corp, 1983Google Scholar
36 Benton AL, Hamsher K: Multilingual Aphasia Examination. Iowa City, AJA Associates, 1983Google Scholar
37 Wechsler D: Wechsler Adult Intelligence Scale Revised (WAIS-R) Manual. Cleveland, Psychological Corp, 1981Google Scholar
38 Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, 2nd ed. Tucson, Ariz, Neuropsychology Press, 1993Google Scholar
39 Nelson HE: A modified card sorting task sensitive to frontal lobe defects. Cortex 1976; 12:313–324Crossref, Medline, Google Scholar
40 Benton AL: Visual perception of line direction in patients with unilateral brain disease. Neurology 1975; 25:907–910Crossref, Medline, Google Scholar
41 Wechsler D: Wechsler Memory Scale—Revised. New York, Psychological Corp, 1987Google Scholar
42 Bridger WH: Cognitive factors in perceptual dysfunction, in Perception and Its Disorders. Edited by Hamburg DA, Pribram KH, Stunkard AJ. Baltimore, Williams & Wilkins, 1970, pp 255–265Google Scholar
43 Buchanan RW, Kirkpatrick B, Heinrichs DW, et al: Clinical correlates of the deficit syndrome in schizophrenia. Am J Psychiatry 1990; 147:290–294Crossref, Medline, Google Scholar
44 Keshavan MS, Sanders RD, Perez G, et al: Diagnostic specificity of the neurologic examination in newly diagnosed psychosis. Am J Psychiatry 2003; 160:1298–1304Crossref, Medline, Google Scholar
45 Sanders RD, McLaughlin N, Pierri JN, et al: Prognostic significance of neurologic examination abnormalities in neuroleptic-naive schizophrenia (abstract). J Neuropsychiatry Clin Neurosci 2000; 12:162Google Scholar
46 Montague LR, Tantam D, Newby D, et al: The incidence of negative symptoms in early schizophrenia, mania and other psychoses. Acta Psychiatr Scand 1989; 79:613–618Crossref, Medline, Google Scholar
47 Braun CMJ, Lapierre D, Hodgins S, et al: Neurological soft signs in schizophrenia: are the related to negative or positive symptoms, neuropsychological performance, and violence? Arch Clin Neuropsychol 1995; 10:489–509Crossref, Medline, Google Scholar
48 Shaji KS: Educational status and neurological abnormalities in schizophrenia. Br J Psychiatry 1991; 158:865–866Crossref, Medline, Google Scholar



