Athymhormia and Disorders of Motivation in Basal Ganglia Disease
Abstract
The author proposes a general model of human motivation as a separate function at the interface between emotion and action, which can be ascribed to subcortical circuits that are mainly centered on a subset of the basal ganglia and on their limbic connections. It is argued that the long-standing historical understatement of the notion of motivation in neurology is not only due to the complexity of the issue, which has proven hard to disentangle from other domains of dysfunction, but also to the persistence of some misleading conceptual orientations in the way neurologists have considered the brain mechanisms of goal-directed action, torn between a nonspecific “activation” view and an exclusively cognitive conception of motivation. How combining early clinical intuitions of some psychiatrists, careful clinical observations of neurological patients, and data derived from experimental studies in animals provide the basis for a coherent model of human motivation and its specific impairment in clinical neurology is explained. Clinical implications that can be drawn from such a model for some neuropsychiatric conditions are proposed.
Among the various topics covered by the young discipline of behavioral neurology, the neurology of motivation probably represents one of the most fascinating and largely unexplored fields of knowledge. It has been only a few years since this issue began to appear in the neurological literature, or at least to be alluded as a specific component of the semiology of such illnesses as stroke, Parkinson’s disease, and Alzheimer’s disease. Nevertheless, it is noteworthy that the topic in itself has not yet taken its place in the classical books of neuropsychology1–3 or even behavioral neurology,4–6 the term motivation being notably absent from the alphabetic index of most of them.
In a recent survey seeking to explore the degree of interest and knowledge of clinicians about this concept,7 British neurologists and psychiatrists underwent a systematic structured interview on “disorders of diminished drive and motivation.” Interestingly, fewer than one half of these specialists believed that these disorders are distinct from depression, and only 52% considered they could indicate damage to the basal ganglia. In their discussion, the authors come to define motivation as a “feeling of ‘owning’ one’s actions and thoughts, an experience often associated to the domain of executive processes,”7 a conception clearly linking motivation to cognition, rather than to emotion. As a conclusion of their paper, the authors recognize that “only recently has the study of motivational processes been extended to integrate biological drives and emotional states in the explanation of purposeful behavior in human beings.”7 As will be developed in the following paragraphs, there is now compelling evidence to consider motivation from the affective rather than cognitive side of neuropsychology.
In the present article, I propose a tentative model of how a subset (i.e., the limbic part) of the basal ganglia, often suspected in animals to subserve motivational functions, also plays the same role in humans. Bilateral lesions of this system may entail profound behavioral changes best described under the term “athymhormia.”
HISTORICAL CONSIDERATIONS
Etymology and Birth of a Neologism
The neologism “athymhormia” was coined in 1922 by two French psychiatrists, Maurice Dide and Paul Guiraud,8 from the privative “a” and two Greek words: ςςμςς, thumos, and ορμη, hormè (from the verb ορμαω, hormao, or ορμειν, hormein, in the infinitive). The first one is well-known by neurologists and psychiatrists, being also used in other neuropsychiatric expressions such as athymia, dysthymia, alexithymia, thymoanaleptic, etc., and means literally “mood, humor” (in the antique sense of liquid constituents of the organism), and more generally “sensations, sentiments, feelings.” The second one, on the contrary, is not commonly found as a Greek root within neuropsychiatric terms. It means “to proceed forward, to rush toward.” (According to the Henry George Liddell and Robert Scott’s Greek-English Lexicon, the term “hormê” has three main meanings: “I. rapid motion forwards, onrush, onset, assault, II. impulse to do a thing, effort, III. in Stoic philosophy, appetition, including reasoned choice and irrational impulse.”) Actually, its significance is even richer, since the term hormè encompasses notions such as impulse, appetite, tendency.9
It is remarkable, as emphasized by Luauté and Saladini,10 that this term began to be used at almost the same time by two other writers: MacDougall,11 who hypothesized, in his “hormic theory,” a properly human intentional process, and independently by the famous neurologist Von Monakow,12 referring to the notion of a “primitive propulsive tendency” of any living being. Obviously, all these scientists were influenced, even if not explicitly, by the vitalist philosophical tradition and the concept of vital impulse (“élan vital”) Henri Bergson13 had put forward as early as in 1907.
The Challenge of a New Terminology
But the coinage of the neologism “athymhormia” probably had a more crucial significance to its authors, once set back in its historical context: it was supposed to characterize the special inhibited behavior of some psychotic patients suffering from a specific form of what started to be called “schizophrenia.” This corresponded to the entity known since its first German description by Kraepelin,14 as “dementia praecox,” an appellation Dide and Guiraud, among other contemporaneous psychiatrists, rejected on the basis of the inappropriate use of the term dementia. Interestingly, at about the same time, another German psychiatrist, Bleuler,15 was about to impose, for the same reasons, but on totally different grounds, the term schizophrenia, putting forward the notion of Spaltung (dissociation). It was precisely to preclude the ascendancy of a “purely psychogenic schizophrenia with blurred contours,” inspired by the psychoanalytic movement, which Dide and Guiraud found “inadmissible,” that they gave such importance to this new terminology.
Beyond the term itself, Dide and Guiraud even though not explicitly, were actually proposing a new syndrome defined by the clinical association of unrelated signs and symptoms, which they ascribed to the dysfunction of a specific brain system.
A New Neuropsychiatric Syndrome
In their description of the fundamental disturbance in patients with dementia praecox, Dide and Guiraud stated, “The disturbance is characterized by an early and precocious fading of the instinctual sources of mental life…the weakening of vital impulse and affectivity being necessary and sufficient conditions to characterize the illness” (“L’affection se caractérise par un fléchissement d’emblée et précoce des sources instinctives de la vie mentale…l’affaiblissement de l’élan vital et de l’affectivité étant l’élément nécessaire et suffisant pour caractériser la maladie.”) Such a description of schizophrenia was not new, indeed, but what was new is its formulation as a syndrome, a fundamental entity which allows clinical recognition of the disease, including “disinterest, inertia, weakening of affective feelings” which they believed central to the occurrence of other expressions of the disease, such as delusions, hallucinations or intellectual and motor disorders. Moreover, and probably more important, as we will see, Dide and Guiraud had an astonishing intuition, some would say premonition, while describing what they thought to be the mechanism of this syndrome: “a selective involution of a group of cells in the sympathetic system located at the level of the pre-optic area and notably including the substantia nigra, but also the putamen and caudate nucleus” (“L’involution élective de groupes de cellules du système sympathique situés au niveau de la région sous-optique et comprenant notamment le locus niger, mais aussi le putamen et le noyau caudé.”) We shall see later how this intuition was close to modern neuroanatomical descriptions, at variance from the dominant conceptions favoring the primacy of the cerebral cortex in all mental functions.
More recently, actually after Dide’s death, his pupil Guiraud pursued his studies along the same line, adding considerations derived from his own observations of patients suffering from Von Economo encephalitis (“encéphalite léthargique”). In his major work, his authoritative “Treatise of Clinical Psychiatry,” Guiraud16 developed the notion of an “hormothymic system,” specifically impaired in schizophrenia, including three components: a first “hormic” component “representing the dynamism of a tendency to satisfy primordial needs,” a second “thymic” component, “provoking specific affective states, pleasant as well as unpleasant” and finally an “effector” component, “endowed with a realizing potential.” Accordingly, athymhormic patients would show impairment at all three of these levels: 1) anhormia considered as the loss of an “instinctual vital dynamism,” 2) athymia, corresponding to the subjective feeling of the same phenomenon and 3) motor inertia which characterizes these patient’s external appearance, and which would be in fact the mere result of the previous two levels of impairment.
In the next paragraphs, I will discuss the relevance of these early—and sometimes seemingly naive—conceptions of the function and dysfunction of a postulated “hormothymic system.” In fact, it seems nowadays that these early intuitions were remarkably pertinent in at least two ways: first as a legacy of the neurological notion of anatomoclinical syndromes, and second, as a brilliant analysis of the complex underpinnings of an as yet uncharted area of the human mental functions.
THE CONCEPT OF ATHYMHORMIA IN NEUROLOGY
In 1988, I and my colleague Michel Poncet17 were struck by the similarity between these classical, almost forgotten, psychiatric notions, and the behavior of two patients we had examined with very comparable ischemic microlesions involving almost “surgically” the head of the caudate nucleus on both sides of the brain, as shown on MRI scans. Since that time, we and others have encountered and reported a number of similar cases, but it seems of interest, here, to briefly describe the clinical history of the two original cases which first attracted our attention.
Athymhormia in Striatal Lesions
First case.
This 64-year-old retired police officer was admitted to the neurology ward for “recent and abrupt behavioral change.” Over the 2 or 3 weeks prior to admission, he had become, according to his spouse, totally apathetic, inactive and prostrate. His medical history was unremarkable except for occasional bouts of elevated blood pressure, which had never justified continuous antihypertensive medication and rare episodes of angina pectoris. On admission, he was clearly hypokinetic with decreased spontaneous movements, facial amimia and Parkinson-like gait. Neurological examination was otherwise normal, except for a moderate limb stiffness. EEG showed mild nonspecific diffuse slowing and CT scan was interpreted as normal for the patient’s age. His general behavior was characterized by a dramatic decrease in spontaneous activity. Totally abulic, he made no plans, showed no evidence of needs, will, or desires. He showed obvious lack of concern about relatives’ as well as his own condition. When questioned about his mood, he reported no sadness or anxiety. Also noteworthy were a loss of appetite (he never asked for food, even if left more than 24 hours without eating) and food preferences (he would eat with the same apparent satisfaction dishes he did or did not like before). Finally, on every instance he was questioned about the content of his mind, he reported a striking absence of thoughts or spontaneous mental activity. Contrasting with these massive behavioral changes, purely cognitive functions seemed relatively spared. On bedside examination, he appeared fully conscious and well oriented. Neuropsychological evaluation was within normal limits, except for tests exploring frontal lobe function (Stroop test, Wisconsin test) which were moderately impaired.
A T2-weighted spin-echo brain MRI scan showed multiple small zones of hyperintensity consistent with the definition of lacunes, located bilaterally in the basal ganglia regions. A single-photon emission tomography with Tc99-HMPAO showed a relative bilateral hypoperfusion in the basal ganglia regions without significant change in cortical (including frontal) blood flow.
Second case.
A 60-year-old university professor, widely respected in his scientific area, first consulted for the specific complaint of “decrease in interest.” His only remarkable medical event was a transient ischemic attack 3 months before and he had no known vascular risk factor. Neurological exam was normal except for moderate slowing of movements and poor spontaneous verbal expression. He had no personal complaint, but his family and professional entourage were struck by a dramatic decrease in activity and motivation. Formerly a hyperactive professional, starting working as soon as 3:00 a.m., he was described by his relatives as a very energetic and motivated person, with high-level investment in his activities, mainly his profession, but also various areas of interest (gardening, reading, fine cuisine, etc.). It was thus clear for anyone who knew him before that something had radically changed in the space of a few weeks. A follow-up of more than 7 years indicated that this radical change remained unaltered during all these years of observation. His own description of his new status was striking: “I just lack spirit, energy. I have no go. I must force myself to get up in the morning, I do things just because I ought to, without any liking or enthusiasm. I have no appetite, no need for eating; I only eat by principle.” During all these years, he occasionally could carry out some of his teaching duty, but only, he said, as a routine. He still used to go to board meetings but never taking the initiative of speaking. During this 7-year follow-up, he never complained of anything, never seemed bored or anxious, showed no sign of depression whatsoever. Remarkably, he never formulated the least question or complaint to his neurologist: actually, during these 7 years he never took the initiative of the conversation. A most embarrassing and impressive situation was his capacity to stay motionless and speechless during endless periods, sitting in front of the examiner, waiting for the first question, totally shut in a profound inertia and passivity, apparently unaware of the bizarreness of the situation. Once the neurologist had posed the first question, he usually answered very appropriately, although briefly. Invariably, the examiner was driven to ask a question which came out almost naturally: “what were you thinking of during all that time? Have you something you would like to say, but you cannot for any reason?.” Invariably, the answer was the same, each time as improbable “No, I’m just thinking of nothing, no idea, no question, no thought at all.” As we will see, this lack of spontaneous mental activity probably pertains to the core feature of the syndrome.
The remainder of the neurological and neuropsychological assessment was unremarkable, with high level of intellectual functioning, normal concentration and visuoconstructional abilities, strictly intact memory, no extrapyramidal signs except poor facial expressiveness, the only significant deficit being on the Wisconsin card sorting, which was markedly impaired, the patient failing to find the first criterion after 50 trials. A brain MRI scan showed multiple bilateral small lacunar infarctions, strictly located to the paraventricular regions, especially damaging the head of the caudate bilaterally. There was neither cortical atrophy, nor white matter changes in the frontal lobes.
Thus, what these two patients had in common was not only this surprising contrast between a profound behavioral change with intact intellectual functioning, but also a very similar lesion pattern, of common origin—lacunar infarcts—which most crucially were strictly restricted to the caudate nuclei. In other terms, this appeared as a new anatomoclinical syndrome, reminding us of the early concept of athymhormia. I will not discuss here the etiological features common to these observations, although it may be of some interest for clinical practice, to concentrate on their contribution to the description of a new neurological entity. Indeed, the same ingredients were present: a striking reduction in spontaneous motion and speech, with subjacent “mental emptiness,” a loss of interest for previously motivating activities and, maybe the crucial point, an apparent flatness or at least poor expressiveness of affect. This latter point had obvious implications: first, from a clinical point of view, that the patients must not be depressed, since depression, even that occurring after cerebral focal lesions,18 is necessarily accompanied with the inner experience of sadness and negative thoughts, which were notably absent in our patients, and second that, beyond an apparent pathology of action, movement or speech, the problem was to be sought clearly elsewhere, probably upstream, at the emotional (i.e., limbic) level.
A Striking Resemblance
A few years earlier, another French group, that of Laplane in Paris, had successively reported before the French Society of Neurology in La Salpêtrière Hospital, two cases of a very similar behavioral syndrome which was ascribed to damage to another subcortical structure: the globus pallidus.19–21 The first case was due to a wasp sting, the other to carbon monoxide poisoning.
At this time, since MRI was not yet available, pallidal involvement was only suspected on CT scan and the exact location of deep brain damage was difficult to ascertain. In both cases however, the clinical features were quite similar, yielding a singular picture of major motor and behavioral inertia and loss of spontaneous mental activity, apparently due to bilateral but disproportionately small brain lesions. These authors mainly focused on a compulsive behavior, mimicking obsessive-compulsive disorder, also present in the same patients (e.g., in one of them an “absolute necessity” to count until reaching a multiple of nine). In fact, such behavior later proved to be nonessential to the diagnosis.
In their first patient, these authors described a total motor inactivity without motor weakness, associated with a very strange state of “mental emptiness,” i.e., absence of spontaneous mental activity, without anxiety or particular suffering, and with otherwise surprisingly intact intellectual capabilities. Discussing the co-occurrence of loss of physical and psychic activity, and chiefly emphasizing the fact that motor as well as intellectual aptitudes were spared, the authors19 hypothesized an impairment of an “auto-activation system for psychic, intellectual and affective life.” According to this view, the basal ganglia would play a dual role in motor and psychic activity in such a way that impairment of this system would suspend spontaneous action as well as mental activity, possibly giving rise to compulsive behaviors. In their following paper,20 they reported a similar case due to carbon monoxide intoxication. Here again, the patient was totally inactive but acted properly on external command, and displayed “pseudo-compulsive” symptoms. In this case, CT scan data were more convincing, showing bilateral pallidal hypodensities, leading the authors to postulate a hitherto unsuspected role for the globus pallidus in psychic self-activation mechanisms. Laplane also pointed out some similarities between this syndrome and various psychiatric conditions such as severe depressive states, obsessive-compulsive disorders and certain forms of schizophrenia.
Shortly after these articles were published, Ali-Chérif et al.22 in Marseille extended Laplane’s findings to three new cases with pallidal lesions,22 all three being due to carbon monoxide intoxication, and focused their description on the comportmental and mental changes in their patients, thus providing a confirmation of the role of the globus pallidus in these aspects of mental functioning. Following is the description given by Ali-Chérif et al. of one of their cases:
The essential semiological finding presented by these two patients consists in a profound inertia which is observable not only in motor activities underlying action on the outer world, but also in mental activity. Action is impaired in its initiation and in maintenance as well. Unless animated by a demand or an order coming from outside, no activity is carried out, even the simplest and the most habitual ones.…Action is also impaired in its progress since it tends to stop unless kept up by external stimulation. Contrariwise, if stimulation is sufficient to give rise to any activity, the latter is always correctly performed.
As an example of the impressive disorder of spontaneous action, they cite the case of the youngest patient of their series, a 19-year-old woman whom her parents had left in the morning on the central square of the small village they were living in, in Corsica, seated on a bench “to have a little rest before getting back home”; several hours later, actually after sunset, she was found exactly at the same place where she had stayed “without budging an inch” during all the day. On another instance, the same patient was found by her parents with heavy sunburns on the beach at the very same place where she laid down several hours before, under an umbrella: intense inertia had prevented her from changing her position with that of the shadow while the sun had turned around.
It must be noted that neither in Laplane’s nor in Ali-Chérif’s reports was any mention made of the terms motivation or motivational disorder. Instead, both authors insisted on the fact that patients were spontaneously inactive and inert but that adequate activity might be obtained from external demands or stimulations, thus pointing out the contrast between impaired self-activation and intact heteroactivation of behavior (“loss of auto-activation”). Also, both authors remarked on the conspicuous similarity between the motor and mental components of the syndrome, whereby the deficit seems to affect both activities inasmuch as they are self-initiated. (Actually, this dichotomy between self- and heteroinitiated acts or thoughts appears not to be totally correct since, in some cases, even external stimulation sometimes fails to yield an appropriate behavior.)
Another aspect pointed out by Ali-Chérif and only marginally alluded to by Laplane, and which may be of considerable importance, is the usual coexistence of impairment in still another domain of mental life, i.e., affect and emotion. “Affectivity is equally impaired, at least in the domain of expression of affects, and this ‘grande indifférence affective’ is regularly pointed out by the patient’s relatives.”22
In fact, it was clear to us that there must exist a purely emotional component to the syndrome, distinct from and probably upstream to its most obvious and directly observable behavioral expression. Along with the observation of new patients, we acquired the conviction that the crucial pathophysiological point was to be sought at the level of emotional regulation and affective processes, as will be discussed below.
AN ANATOMY-DRIVEN ANALYSIS OF THE SYNDROME
Whatever the case, the connection between the two groups of observations led us to the following reasoning: if a similar neurobehavioral picture can result from two separate lesion sites, this must indicate the disruption of a neural system which would include these two brain sites. Obviously, a plausible candidate could be found among the frontal-striatal-pallidal loops, especially the “limbic loop,” given the conviction we had to deal with a syndrome more or less closely linked to the emotional brain.
Evidence of a Specific Limbic Connection Between Striatal and Pallidal Regions
According to the now-classical neuroanatomical modelling of the connections between the basal ganglia and frontal cortex (see for example Cummings23), it is well recognized that cortico-subcortical connections are organized in several loops functioning in parallel, each presumably subserving a specific function. Alexander et al.24–26 described five parallel cortico-subcortical loops originating from and terminating in different parts of the frontal cortex. One of these circuits, mainly originating in anterior cingulate cortex, encompasses the ventral striatum, the ventral part of the globus pallidus and the posteromedial medial-dorsal nucleus of the thalamus, and projects back again to the anterior cingulate (Figure 2). This circuit, connecting cortical and subcortical components of the limbic system, is very similar to the “limbic loop” postulated by Nauta27 to be involved in motivational and emotional control in the rat. There is considerable, converging evidence to consider the limbic (or ventral) striatopallidum as an “interface between motivation and action”28 or as the site of “conversion of motivational processes into behavioral output.”29 Such conceptions are logically derived from the observation that the limbic striatopallidum receives afferents from the amygdala, involved in emotional labeling of sensory stimuli, and from the hippocampal formation, presumed to compare incoming information with past experience, and projects to the rest of the basal ganglia mass, involved in initiating and organizing motor acts.30,31
Experimental Evidence
Such a role in converting affective information into motivated acts was first suggested from the classical self-stimulation experiments in rats, and various subsequent manipulations of this model have shown a major participation of the ventral striatum, recipient of dopaminergic afferents, in such behavior. For instance, direct infusion of dopaminergic agents in the nucleus accumbens alters considerably the animal’s approach behaviors,32 whereas rats allowed to self-stimulate with autoinjections of amphetamines in the accumbens develop a strong tendency to autostimulate. Various other interventions on the dopamine system within the accumbens have shown alterations of stimulating effects of rewarding stimuli on the animal’s behavior.33,34 Studies of monkeys indicate that dopamine projections from the ventral tegmental area of the midbrain to the nucleus accumbens of the ventral striatum fire selectively in response to presentation of reward cues.35 Rolls et al. (see Rolls36) recorded a large number of neurons in the limbic striatum and showed that they can react to such various conditions as novel stimuli, various previously reinforced visual stimuli, or even cues announcing the beginning of the task. Other studies also suggest that dopamine release occurs more robustly in the accumbens during reward anticipation than during reward consumption.34,37 Recent brain functional imaging studies have largely confirmed the role of the ventral striatum including nucleus accumbens in rewarding situations in humans. For example, using a money reward paradigm with event-related functional MRI, Knutson et al.38 have confirmed the specific involvement of ventral striatum in reward anticipation, whereas a ventromedial cortical frontal area is activated upon reward outcome. The same authors39 have further demonstrated that ventral striatum activation elicited by reward is proportional to the magnitude of reward, but not to punishment.
Data concerning the role of the globus pallidus are more sparse but recent work has also shown the implication of pallidal neurones in reward mechanisms.40,41 Electrophysiological studies of animals have thus implicated ventral pallidum activation as a correlate of food reward, cocaine reward, and brain stimulation reward.42,43 In humans, electrical stimulation close to the ventral pallidum may induce bouts of affective mania that can last for days.44
The exact nature and function of the ventral pallidum remain mysterious. It seems that this point of convergence of striatal fibers plays the role of concentrating in a few groups of neurons an assembly of afferents containing the relevant information about the nature of rewarding stimuli. The ventral pallidum thus receives a dense enkephalinergic projection from the nucleus accumbens and has been implicated as a locus mediating the rewarding and reinforcing effects of psychostimulant and opiate drugs. It may play the special role of funneling limbic afferents into the mediodorsal part of the thalamus and other subcortical efferents of the ventral striatopallidal system. From animal experiments using taste reactions to sweetness, Berridge45 considers the ventral pallidum as “the only distinct brain structure to be necessary for generating a normal positive affective reaction to a sweet or otherwise pleasant taste.” Whether or not this specificity applies to other positive stimuli remains unknown.
ATHYMHORMIA AND THE BASAL GANGLIA: FURTHER EVIDENCE
Since our initial observations, several series of focal lesions involving the basal ganglia have globally confirmed the high frequency of motivational disorders in striatal and, to a lesser degree, pallidal lesions.46–50 Most of these studies, however, did not provide detailed discussion of the underlying mechanisms.
For example, a recent series of patients with caudate vascular lesions emphasized abulia, defined as “decreased spontaneous activity, prolonged latency in responding to questions, fatigue, and an aversion to any activity.”50 Abulia was reported in 15 out of 25 cases and appeared as the most frequent neurological abnormality in these patients. Contrary to the athymhormia case reported above, some of these patients had unilateral lesions, challenging the view that the striatolimbic disconnection has to be bilateral to entail severe motivational disorders. However, it is possible that motivation disorders in unilateral caudate lesions are only transient, as opposed to severe persistent disorders in bilateral cases. In the same paper, the authors seem to differentiate abulia from “psychia akinesia,” found in three patients and defined as “severe impairment of mental and psychic activity and loss of affective and motor response to external stimuli.” Obviously, this distinction seems somewhat arbitrary since the difference might be quantitative rather than qualitative.
Worthy of note is that in primates the limbic (mainly cingulate) afferents to the striatum, unlike those in rodents, are not restricted to its ventral part—the accumbens nucleus—but are represented on a broader part of the caudate itself, along a strip extending rostrocaudally on the mesial aspect of the nucleus.51 This could explain, in humans, the occurrence of athymhormia with caudate lesions apparently sparing the more ventrally located accumbens nucleus. Recent brain imaging studies (e.g., Knutson et al.39) have further suggested that accumbens and medial caudate may activate in different rewarding contexts, with possibly individual variations in laterality.
Finally, the now well-documented demonstration of a circuit centered on the basal ganglia, probably involved in the “process of converting motivation into action” suggests that the human syndromes reported above as consequences of striatal or pallidal lesions would result from bilateral disruption, at various levels, of this circuit. If this assumption is correct, athymhormia should be possibly associated with 1) nonsymmetrical striatopallidal lesions and 2) bilateral damage to the loop outside the striatopallidum.
A first argument in favor of our view is provided by observations in which the same syndrome results from asymmetrical lesions of this system. Several such observations are available in the recent literature. For instance, Bellmann and Assal52 have reported a clinically very similar case of athymhormia with a large infarct of the caudate head on one side, and a pallidal lesion on the other hemisphere (Figure 3). In a case of Moya-Moya syndrome with typical athymhormia,53 we found a similar anatomical pattern: caudate lesion on one side, medial pallidal on the other side.
Other Sites of Lesions Possibly Responsible for Athymhormia
Even more interesting for the present discussion are cases of athymhormia or motivational disorders occurring after lesions outside the striatopallidal complex. Indeed, it would stand as a strong argument in favor of our hypothesis that Nauta’s limbic loop is the neural substrate of human motivation if one could demonstrate similar clinical features from lesions in other sites of the loop. More specifically, our hypothesis was that damage at any point of the loop, provided that it disrupts the system bilaterally, is susceptible of provoking similar behavioral changes.
Actually, although the purest form of the syndrome would be completed after damage to the limbic striatopallidum, which seems to constitute the center of the system, lesions damaging the white matter fibers linking the limbic frontal cortex to anterior striatum could have the same consequences as lesions to the striatum itself. Likewise, lesions situated at the output side of the system (i.e., paramedian “limbic” nuclei of the thalamus and thalamocortical afferents) would also yield a similar syndrome.
Disconnection of the Ventral Striatum From Frontal Afferents
Although the ventral striatum, including the accumbens, mainly receives fibers from the medial frontal cortex54 its afferents also include several other paralimbic and periallocortical structures (hippocampal formation, entorhinal cortex, and olfactory cortex), as well as proisocortical areas (such as agranular insular and perirhinal cortices).55 It is thus probable that cortico-subcortical lesions, such as those resulting from severe brain trauma, will result in more or less complete disconnection of the anterior striatum from its cortical afferents. Patients with bilateral orbitofrontal damage usually show disinhibited behavior, sometimes with mania-like episodes rather than the opposite features of apathy and absence of spontaneous actions described in athymhormia. However, it is this author’s experience that both behavioral changes may coexist, either in temporal succession, or even simultaneously, giving rise to a curious association of profound loss of interest and motivation on one hand, with excessive familiarity and lack of compliance to social or conventional rules.
The effect of cingulate or paracingulate lesions on motivation is well established. Apathy is a classical symptom of damage to cingulate cortex and consequences of the classical cingulectomy, a surgical intervention proposed to alleviate the symptoms of severe intractable anxiety or pain, have been widely documented.56,57 The most anterior and rostral part of the cingulate, projecting precisely on the accumbens nucleus, has been labeled as the “affective cingulate,” as opposed to more posterior cortex, “cognitive cingulate,” which projects on more lateral parts of the striatum.57 Cingulate lesions are mostly vascular or surgical. Loss of initiative and spontaneous action, decreased interest and drive, and impoverishment of affective life58,59 are usually described. In surgical cases, the presence of the underlying psychiatric illness, however, renders an interpretation difficult. In spontaneous pathology, cingulate lesions always occur in association with generally severe cognitive, attentional and memory disturbances, which, obviously, will also obscure more or less completely the impairment in motivation and drive.
I have reported elsewhere60 clinicoanatomical data in a patient who first sustained an isolated right caudate infarct and several years later a probably watershed infarct in the opposite mesial frontal white matter (Figure 3). In this case, the frontal-striatal-pallidal circuit was interrupted at a different level in each hemisphere. The athymhormia symptoms occurred after the second ischemic event, suggesting that mesial frontocaudate fibers were compromised by the latter event, and thereby confirming the inclusion of these frontocaudate connections as part of the postulated brain substrate of human motivation.
Bilateral Disruption of the Output of the Striatopallidal System
In the same vein, one should expect lesions compromising the output of the system to entail the same consequences as damage to the core part of the system itself. Accordingly, paramedian mesencephalo-diencephalic infarcts have been reported to give rise to apathetic and unmotivated behavior.61,62 In the latter case, a bilateral infarct in the territory of the mamillothalamic arteries, a brain metabolic exploration with SPECT demonstrated a decrease in frontal blood flow, unlike in our cases of caudate lesions. Actually, disturbances of action and motivation are often present in cases of bilateral medial thalamic lesions, but, here again, interpretation of behavioral changes is generally obscured by the co-occurrence of severe memory and cognitive impairment. In a recent well-documented case report,63 two small infarcts involving bilaterally the mediodorsal thalamic nuclei have resulted in typical symptoms of profound athymhormia. After a coronary arteriography, this 58-year-old man suddenly developed a left hemiparesis and coma. After recovery from the initial stage, he was found with profound behavioral changes:
Instead of the active man he used to be, he had become an apathetic, passive, and indifferent person who seemed to have lost all emotional concern and initiative. His affect was clearly blunted. The patient remained indifferent to visitors or when he received gifts. He did not show any concern for his relatives or his illness and manifested no desire, no complaint, and no concern about the future. The patient, who used to be a successful traveling salesman, had entirely lost concern about his business. There was a striking absence of thoughts and spontaneous mental activity. He rarely spoke spontaneously and took no verbal initiative. When asked about the content of his thoughts, the patient claimed he had none, suggesting a state of mental emptiness. Unless encouraged by the hospital staff or his relatives, he did not initiate any activity. When external stimulation disappeared, any induced activity was immediately interrupted. Every morning, the patient stayed in bed until he was encouraged to rise and get dressed. Once dressed, he returned to bed again or sat down in an armchair for the entire day. He moved very little unless urged to do so and reverted to his habitual state of athymia once he was left alone. There were no symptoms of depression. No stereotyped activities were observed.63
This description is unquestionably very similar to what we called “athymhormia” in our striatal patients. A dramatic decrease in spontaneous actions and intentions, “blunted affect” and mental emptiness were clearly demonstrated. A brain MRI scan (Figure 4) demonstrated very clearly the two small infarcts located in the medial thalamic region, corresponding to the paramedian arteries territory. Interestingly, impairment of cognitive functions (memory, abstract reasoning, etc.) which was present at the beginning of the evolution, gradually disappeared during the first 12 months. However, the athymhormic syndrome remained intact. This observation suggests that, if some (especially cognitive) symptoms result from disconnection or deactivation of the frontal cortex, athymhormia cannot be explained in such terms. Accordingly, a brain scintigraphy with HMPAO showed very surprisingly and most notably two regions of hypoperfusion, one in the caudate nucleus, the other projecting on the medial frontal cortex, a pattern which persisted throughout the 12 months of follow-up. As emphasized by these authors, this result strongly supports our interpretation in terms of disconnection of a bilateral network successively including cortical, striatal, pallidal and finally thalamic stages. The SPECT/MRI comparison in their case further suggests that disruption of this neural network at one point alters the level of neural activity at both the previous and following stages of the system. One may speculate that more refined functional imaging methods, such as fMRI, would have shown similar involvement at the pallidal stage, which may remain beyond the spatial resolution of SPECT.
MECHANISMS OF ATHYMHORMIA: CORTICAL DEACTIVATION OR BASAL GANGLIA DISCONNECTION?
One important issue to be dealt with at this point of the discussion is that of the brain mechanism underlying the observed dysfunction in athymhormia. Specifically, two main opposing views may be put forward.
The first one is probably the more commonly found in the classical neurological literature. Adams and Victor,64 under the term “apathy,” referred to “a quantitative reduction of all activities,” not unlike akinetic mutism, and ascribed both conditions to the impairment of a “centrencephalic-cortical energetic mechanism.” They further proposed to isolate, among this group, patients designated as “hypobulic,” showing a lesser degree of the same defect, with “apathy, indifference, loss of interest and shallowness of thinking.” Miller Fisher65 prefers the term “abulia minor,” considering the syndrome, here again, as a minimal form of akinetic mutism. In an often quoted article, he clearly describes this pattern of behavioral changes in neurological patients as “lack of spontaneity of action, of speech and lack of initiative” emphasizing principally the motor and mental slowness of the patients. Moreover, “the patients have a reduced flow of thoughts, they are uncomplaining and express little in the way of needs or satisfaction.…Reaction to loved ones is slight, and tearfulness is in abeyance. Temper outbursts and anger are absent.” Indeed, such description is quite reminiscent of the above reported striatopallidal patients. Yet, Fisher’s interpretation remains that of a reduced form or a transient state of akinetic mutism, as a result of “failure of a basic primitive activating or facilitating system.” Accordingly, all the anatomical discussion lies upon the classical interpretation of akinetic mutism, as possibly resulting from midline lesions, from the mesencephalon to the medial frontal cortices. Almost naturally, this conception leads to the notion of an ascending activating system, much akin to the reticular system and its role in wakefulness and cortical activation.
Laplane advocated the same kind of interpretation in his accounts of patients with “loss of psychic auto-activation.” Based on results of PET studies in bipallidal patients, Laplane66 suggested that “loss of self-activation” observed in pallidal lesions results from a mechanism of deactivation of the frontal cortex, since patients in their series had (moderate) frontal hypoperfusion.67 The role played by the basal ganglia would then be considered tantamount to that of a non specific activation system. Along the same line of reasoning, Trillet et al.,46 in their report of three cases of caudate infarcts with persistent apathy, flattened affect, and lack of interest and initiative, discussed the function of the caudate nuclei in spontaneous motor activity as a regulator of prefrontal cortex activity. Actually, Fisher, as well as Laplane, or Trillet share in common the same conception of the brain substrate of mental processes that implicitly comprises a necessary cortical final stage. This cannot accommodate well the notion of a separate motivation system involving specific neural structures and mechanisms. Yet accumulating evidence, both clinical and experimental, seems nowadays sufficient to strongly suspect the reality of an “hormothymic system,” as proposed half a century ago by Guiraud, as the evolutionary mark of an universal motivational apparatus, necessary to species survival throughout the animal kingdom.
Finally, it seems relevant to cite here work by Marin,68–70 who points to apathy as the specific manifestation of lack of motivation in neurological and psychiatric patients, defined as 1) diminished goal-directed overt behavior (lack of initiative and perseverance, etc.), 2) diminished goal-directed cognition (lack of interest, lack of concern, etc.), 3) diminished emotional concomitants of goal-directed behavior (unchanging affect, etc.). The originality of the position defended by Marin is that, thus defined, apathy is a syndrome that can result from various conditions, in psychiatry as well as in neurology. Although the concept is very close to that of athymhormia, especially as regards the three-level analysis of the syndrome, there was no systematic attempt at relating apathy to the dysfunction of a specific brain system.
FROM AFFECT TO ACTION: “ATHYMHORMIA” AS A STRIATAL-LIMBIC DISCONNECTION SYNDROME
Conceiving the disorder as a fundamental defect in converting past or present emotional experience into an actual action renders an account in terms of cortical activation clearly insufficient. Instead, one must rather conceive the relevant mechanism as supported by a neural network interfacing on one hand systems apt to analyze, maintain in long-term memory and retrieve the affective value of a given stimulus or contextual cue (a process presumably subserved by the amygdala-hippocampus-orbital-frontal system) and, on the other hand, systems in charge of controlling movement initiation, mental activity and emotional expression (possibly represented in various parts of the basal ganglia, under the form of separate frontal-subcortical loops).
Figure 5 depicts a speculative model of the brain mechanisms underlying motivation in man. In the light of theories derived from animal research, motivation may appear as the behavioral consequence of the chain of events starting from the association between a stimulus and its affective significance for the animal71,72 and resulting in an overt action, either approaching the stimulus (in case of an appetitive or rewarding stimulus) or avoiding it (in case of an aversive or punishing stimulus). Not unlike classical Pavlovian conditioning, repetition of such associations over time would result in some kind of conditioning, which determines goal-directed behaviors.
From an anatomical point of view, there is strong evidence that the amygdala is the structural site of association between stimulus and reward.71 The strong projections from amygdala to the limbic striatum and connections between limbic and motor parts of the striatum suggest for the limbic striatopallidum the role of converting affective representations contained in the amygdala into motor programs: supplied with dopamine by specific dopamine afferents, which act as the energizing “fuel” of the system, the limbic part of the striatum would represent the driving center of the 3 main frontostriatal loops functioning as three articulated gears turning together. Continuous functioning of the motor and associative loops would be dependent on the functioning of the limbic one, with “energy” being provided by their respective dopaminergic input. Each loop possesses a specific output (motor acts for the motor loop, emotional expression for the limbic one and spontaneous mental activity for the associative one), accounting for the three main symptoms of the athymhormic syndrome. According to this representation, damage to the limbic loop, while leaving intact the other two, would result in impaired spontaneous cognitive and motor activity, giving rise to the characteristic symptoms of athymhormia: lack of spontaneous action (but intact motor functioning) and poverty of spontaneous thinking (but relatively preserved intellectual capacities). This model provides a plausible framework for the curious association between loss of motor and mental activity, as well as their reversibility upon external stimulation. According to the model, however, blunting of emotional expression would be, unlike the other two symptoms, a nonreversible phenomenon, but this does not preclude possible preservation of emotional experience itself.
CONCLUSIONS
To complete this article, it seems potentially useful to suggest a few practical implications for the clinician, either neurologist, psychiatrist or any professional interested in the behavioral consequences of brain lesions. One first observation is that motivation disorders, provided that they are specifically searched for, are probably much more common than the few number of publications about the specific topic would indicate. The reason is probably that in most cases, athymhormia or milder forms thereof, contrary to the above described relatively pure cases, is much more often associated with other cognitive deficits, especially memory, attentional, or other behavioral changes. As we have seen, such associations can be found in focal lesions, inasmuch as they damage decisive structures, such as the medial thalamus or medial frontal subcortical fibers. More often, however, they occur in the context of dementia, not only vascular dementia where abrupt onset of the syndrome allows early and relatively easy recognition, but also in degenerative dementia, where onset is more insidious and the symptoms intermingled with memory and attentional impairment. In the recent literature on dementia and cognitive disorders in the elderly, motivational disorders are often referred to under the term “apathy,” a concept widely used in recent analyses of behavioral problems in neurology, in particular in degenerative disease.73–76 In Alzheimer’s disease, apathy is gaining increasing interest from clinicians not only for its as yet underestimated frequency, but also for its potential use in differential diagnosis between dementia and normal aging. Likewise, considerable recent work has been devoted to the issue of differentiating apathy from depression, a topic still subject to discussion.76,77 At first sight, the two conditions may appear to share common features: decreased activity, hypokinesia, lack of goals and perspectives, hypersomnia in some cases. In fact, they are basically different from several points: first, in athymhormic patients examination fails to disclose any sign of sadness or negative thoughts. Instead, patients are more or less—sometimes dramatically—indifferent to any environmental event as well as to their own condition. Moreover, the mental emptiness demonstrated in these patients is diametrically opposite to the classical negative “rumination” of depressed patients. It is thus of primary importance to specifically investigate the actual content of patients’ spontaneous thought and mood, to reach the appropriate diagnosis. This distinction does possess important implications for forensic purpose, where experts are often required to decide whether behavioral symptoms (e.g., loss of interest and decreased activity after brain traumatic lesions) are or are not the direct consequence of the initial incident. Finally, evidence of bilateral involvement of the limbic loop will provide crucial support to determining the degree of imputability.
In so-called frontotemporal dementia, behavioral changes are usually more pronounced and occur earlier than memory and cognitive changes. From the behavioral point of view, it has been proposed to separate apathetic versus disinhibited forms of frontotemporal dementia.78 In the apathetic form, loss of activity and blunted affect are prominent, and frontal atrophy and hypometabolism are global (as opposed to localized to orbital regions in the disinhibited form), suggesting that medial frontal involvement has “masked” the effects of orbital involvement. In such “cortical dementias,” even though basal ganglia may not be intact, behavioral disorders are traditionally chiefly associated with cortical involvement.
Among more strictly subcortical degenerative diseases, three related conditions—Lewy Body disease, Parkinson disease and Parkinson disease with dementia—are currently considered to share common mechanisms.73 But the profile of behavioral disorder is rather one of delusions and hallucinations than of apathy and flattened affect. Actually, it seems that striatal dysfunction in this group is not directly responsible for behavioral changes. Moreover, motor deficits would possibly prevent the observation of a decrease in motivation. On the contrary, apathy is classically reported in two other diseases, progressive supranuclear palsy79 and Huntington’s disease.80 In both cases, although cognitive involvement is often prominent, the symptoms of loss of interest, inactivity and flattened affect are clearly recognizable. In progressive supranuclear palsy, in particular, lesions and symptoms are very similar to those of postencephalitic parkinsonism, which has served as a model of apathetic behavior. In one case of progressive supranuclear palsy, Destee et al.81 have related the prominent athymhormic symptoms to the particularly severe lesions observed postmortem in the globus pallidus. Finally, pallidal surgical lesions performed for intractable parkinsonism have given rise to behavioral changes very similar to that of spontaneous pallidal or striatal lesions, contributing to make this surgery undesirable.82
Obviously, these various pathological conditions do not contribute equally to our comprehension of the neural basis of human motivation. It is possible that in a near future, most significant advances may result from studies in psychiatric disease, especially schizophrenia, where basal ganglia involvement has been suspected in several instances.83–86 It would, then, be only fair that the notion of athymhormia, born out of the intuition of two psychiatrists, and which has so much contributed to neurology, could, in return, open the way to a neurological comprehension of schizophrenia.
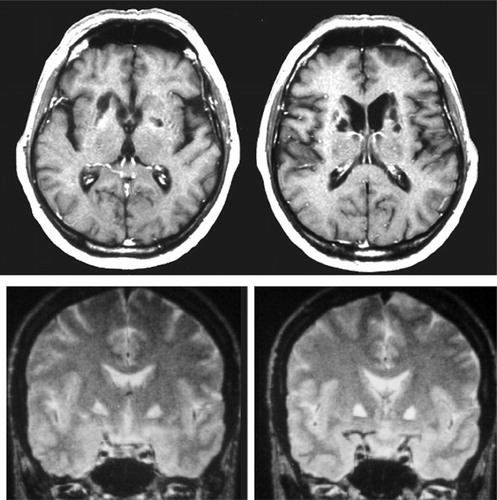
FIGURE 1. The Two Main Lesion Sites Provoking Pure Motivational Disorders in Humans: Head of the Caudate Nuclei (Top) and Medial Globus Pallidus (Bottom)
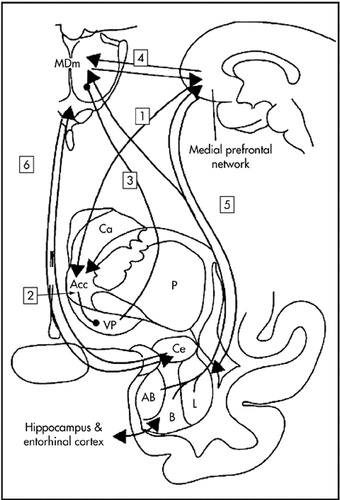
FIGURE 2. The Limbic Frontal-Striatal-Pallidal Loop of Nautaa
a1: medial prefrontal cortex to nucleus accumbens (acc); 2: nucleus accumbens to ventral pallidum (vp); 3: ventral pallidum to medial dorsal nucleus (medial part) of the thalamus (MDm); 4: thalamocortical interconnections; 5: amygdala-medial-frontal connections; 6: amygdala-medial-thalamus connections.
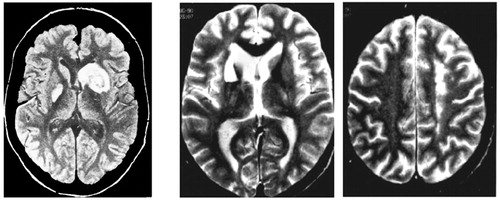
FIGURE 3. Two Cases of Severe Athymhormia Resulting From Asymmetrical Subcortical Lesionsa
aIn both cases, it is hypothesized that motivational disorders were due to bilateral disruption of the frontal-striatal-pallidal limbic loop. Left: caudate (right) and pallidal (left) lesions (from Bellmann and Assal52 with permission). Center, Right: caudate (left) and precingulate (right) lesions.
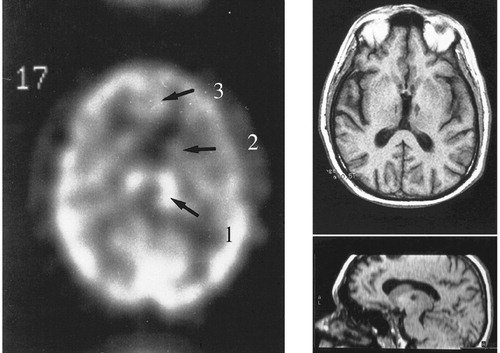
FIGURE 4. A Case of Severe Athymhormia Due to Bilateral Medial Thalamic Lesionsa
aAnatomical findings on MRI scan (right). HMPAO single photon emission showing, at the initial stage, luxury perfusion of the infarcted areas,1 with hypoperfusion of caudate,2 and medial frontal cortex.3 With permission from Engelborghs et al.63
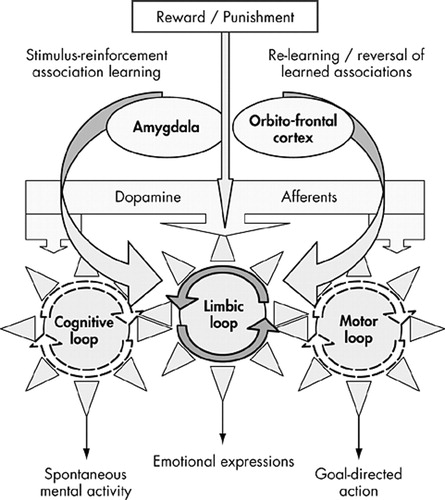
FIGURE 5. The Limbic Loop: An Interface Between Emotion and Actiona
aAt the emotional level (top part of the figure), the stimulus reinforcement association, which takes place in the amygdala, continuously reformulated and evaluated by the orbitofrontal cortex, determines goal-directed behaviors (bottom of the figure) by informing the ventral striatum (including accumbens), which drives the rest of the system by a mechanism best represented by interlinked gears. The dopaminergic afferents would play the role of “fueling” the system.
1 Hécaen H, Albert ML: Human Neuropsychology. New York, John Wiley & Sons, 1979Google Scholar
2 Heilman KM, Valenstein E (eds): Clinical Neuropsychology. New York, Oxford University Press, 1979Google Scholar
3 Vinken PJ, Bruyn GW, Klawans HL, et al. (eds): Clinical Neuropsychology. New York, Elsevier, 1985Google Scholar
4 Mesulam M-M (ed): Principles of Behavioral Neurology. Philadelphia, FA Davis, 1985Google Scholar
5 Feinberg TE, Farah MJ (eds): Behavioral Neurology and Neuropsychology. New York, McGraw-Hill, 1997Google Scholar
6 Kirshner HS: Behavioral Neurology: Practical Science of Mind and Brain. Boston, Butterworths, 2002Google Scholar
7 Vijayaraghavan L, Krishnamoorthy ES, Brown RG, et al.: a delphi survey of British neurologists and psychiatrists. Mov Disord 2002, 17:1052–1057Google Scholar
8 Dide M, Guiraud P: Psychiatrie du médecin praticien. Paris, Masson, 1922Google Scholar
9 Siegel RE: Galen on Psychology, Psychopathology, and Function and Diseases of the Nervous System. Basel, Switzerland, S Karger, 1973Google Scholar
10 Luauté J-P, Saladini O: Le concept Français d’athymhormie de 1922 à nos jours. Rev Can Psychiatr 2001; 46:639–643Crossref, Medline, Google Scholar
11 MacDougall W: An Outline of Psychology. London, Methuen, 1928Google Scholar
12 von Monakow C, Mourgue R: Introduction biologique à l’étude de la neurologie et de la psychopathologie. Paris, Alcan, 1928Google Scholar
13 Bergson H: L’évolution créatrice. Paris, Alcan, 1907Google Scholar
14 Kraepelin E: Dementia Praecox and Paraphrenia. Translated by Barclay RM; edited by Robertson GM. Edinburgh, E & S Livingstone, 1919Google Scholar
15 Bleuler E: Dementia Praecox or the Group of Schizophrenias (1911). Translated by Zinkin J. New York, International Universities Press, 1950Google Scholar
16 Guiraud P: Psychiatrie clinique. Paris, Le François, 1956Google Scholar
17 Habib M, Poncet M: Perte de l’élan vital, de l’intérêt et de l’affectivité (syndrome athymhormique) au cours de lésions lacunaires des corps striés. Rev Neurol 1988; 144:571–577Medline, Google Scholar
18 Gainotti G, Azzoni A, Marra C: Frequency, phenomenology and anatomical-clinical correlates of major post-stroke depression. Br J Psychiatry 1999; 175:163–167Crossref, Medline, Google Scholar
19 Laplane D, Widlocher D, Pillon B, et al.: Comportement compulsif d’allure obsessionnelle par nécrose circonscrite bilatérale pallido-striatale: encéphalopathie par piqure de guêpe. Rev Neurol 1981; 137:269–276Medline, Google Scholar
20 Laplane D, Baulac M, Pillon B, et al.: Perte de l’auto-activation psychique: activité compulsive d’allure obsessionnelle: lésion lenticulaire bilatérale. Rev Neurol 1982; 138:137–141Medline, Google Scholar
21 Laplane D, Baulac M, Widlocher D, et al.:. Pure psychic akinesia with bilateral lesions of the basal ganglia. J Neurol Neurosurg Psychiatry 1984; 47:377–383Crossref, Medline, Google Scholar
22 Ali-Chérif A, Royère ML, Gosset A, et al.: Troubles du comportement et de l’activité mentale après intoxication oxy-carbonée: lésions pallidales bilatérales. Rev Neurol 1984; 140:401–405Medline, Google Scholar
23 Cummings JL: Frontal-subcortical circuits and human behavior, Arch Neurol 1993; 50:873–880Google Scholar
24 Alexander GE, Crutcher MD: Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurol Sci 1990; 13:266–271Crossref, Medline, Google Scholar
25 Alexander GE, Crutcher MD, DeLong MR: Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” function. Prog Brain Res 1990; 85:119–146Crossref, Medline, Google Scholar
26 Alexander GE, DeLong MR, Strick PL: Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9:357–381Crossref, Medline, Google Scholar
27 Nauta WJH: Circuitous connections linking cerebral cortex, limbic system, and corpus striatum, in The Limbic System: Functional Organization and Clinical Disorders. Edited by Doane BK, Livingston KE. New York, Raven Press, 1986, pp 43–54Google Scholar
28 Mogenson GJ, Jones DL, Yim CJ: From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 1980; 14:69–97Crossref, Medline, Google Scholar
29 Apicella P, Ljundberg T, Scarnati E, et al.: Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res 1991; 85:491–500Crossref, Medline, Google Scholar
30 Salamone JD: The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res 1994; 61:117–133Crossref, Medline, Google Scholar
31 Schultz W: The primate basal ganglia: between the intention and outcome of action, in Functions of the Cortico-Basal Ganglia Loop. Edited by Kimura M, Graybiel AM. Tokyo, Springer, 1995Google Scholar
32 Ikemoto S, Panksepp J: Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav Neurosci 1996; 110:331–345Crossref, Medline, Google Scholar
33 Everitt BJ, Robbins TW: Amygdala-ventral striatal interactions and reward-related processes, in The Amygdala. Edited by Aggleton JP. Chichester, UK, John Wiley & Sons, 1992, pp 401–430Google Scholar
34 Ikemoto S, Panksepp J: The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev 1999; 31:6–41Crossref, Medline, Google Scholar
35 Schultz W, Tremblay L, Hollerman JR: Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex 2000; 10:272–283Crossref, Medline, Google Scholar
36 Rolls ET: A theory of emotion and consciousness, and its application to understanding the neural basis of emotion, in The Cognitive Neurosciences. Edited by Gazzaniga MS. Cambridge, Mass, MIT Press, 1995, pp 1091–1106Google Scholar
37 Berridge KC, Robinson TE: What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 1998; 28:309–369Crossref, Medline, Google Scholar
38 Knutson B, Fong GW, Adams CM, et al.: Dissociation of reward anticipation and outcome with event-related FMRI. Neuroreport 2001; 12:3683–3687Crossref, Medline, Google Scholar
39 Knutson B, Adams CM, Fong GW, et al.: Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 2001; 21:RC159Google Scholar
40 McAlonan GM, Robbins TW, Everitt BJ: Effects of medial dorsal thalamic and ventral pallidal lesions on the acquisition of a conditioned place preference: further evidence for the involvement of the ventral striatopallidal system in reward-related processes. Neuroscience 1993; 52:605–620Crossref, Medline, Google Scholar
41 Panagis G, Miliaressis E, Anagnostakis Y, et al.: Ventral pallidum self-stimulation: a moveable electrode mapping study. Behav Brain Res 1995; 68:165–172Crossref, Medline, Google Scholar
42 Gong W, Neill D, Justice JB Jr: 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain Res 1997; 754:103–112Crossref, Medline, Google Scholar
43 McBride WJ, Murphy JM, Ikemoto S: Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res 1999; 101:129–152Crossref, Medline, Google Scholar
44 Miyawaki E, Perlmutter JS, Troster AI, et al.: The behavioral complications of pallidal stimulation: a case report. Brain Cogn 2000; 42:417–434Crossref, Medline, Google Scholar
45 Berridge KC: Pleasures of the brain. Brain Cogn 2003; 52:106–128Crossref, Medline, Google Scholar
46 Trillet M, Croisile B, Tourniaire D, et al.: Disorders of voluntary motor activity and lesions of caudate nuclei. Rev Neurol (Paris) 1990; 146:338–344Medline, Google Scholar
47 Caplan LR, Schmahmann JD, Kase CS, et al.: Caudate infarcts. Arch Neurol 1990; 47:133–143Crossref, Medline, Google Scholar
48 Cummings JL: Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50:873–880Crossref, Medline, Google Scholar
49 Bhatia KP, Marsden D: The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 1994; 117:859–876Crossref, Medline, Google Scholar
50 Kumral E, Evyapan D, Balkir K: Acute caudate vascular lesions. Stroke 1999; 30:100–108Crossref, Medline, Google Scholar
51 Selemon LD, Goldman-Rakic PS: Longitudinal topography and interdigitation of corticostriatal projections in the Rhesus monkey. J Neurosci 1985; 5:776–794Crossref, Medline, Google Scholar
52 Bellmann A, Assal G: Les multiples propos d’une athymhormique. Rev Neuropsychol 1996; 6:101–120Google Scholar
53 Milandre L, Habib M, Royere ML, et al.: Syndrome athymhormique par infarctus striato-capsulaire bilatéral: maladie de Moya-Moya de l’adulte. Rev Neurol 1995; 151:383–387Medline, Google Scholar
54 Herrero M-T, Barcia C, Navarro JM: Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst 2002; 18:386–404Crossref, Medline, Google Scholar
55 Nakano K: Neural circuits and topographic organization of the basal ganglia and related regions. Brain Dev 2000; 22:S5-S16Google Scholar
56 Devinsky O, Morrell MJ, Vogt BA: Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118:279–306Crossref, Medline, Google Scholar
57 Paus T: Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev 2001; 2:417–424Crossref, Google Scholar
58 Stuss DT, Benson DF: Emotional concomitants of psychosurgery, in Neuropsychology of Human Emotion. Edited by Heilman KM, Satz P. New York, Guilford, 1983, pp 111–140Google Scholar
59 Damasio AR, Van Hoesen GW: Emotional disturbances associated with focal lesions of the limbic frontal lobe. Ibid, pp 85–110Google Scholar
60 Habib M: Apathie, aboulie, athymhormie: vers une neurologie de la motivation humaine. Rev Neuropsychologie 1998; 8:537–586Google Scholar
61 Katz DI, Alexander MP, Mandell AM: Dementia following strokes in the mesencephalon and diencephalon. Arch Neurol 1987; 44:1127–1133Crossref, Medline, Google Scholar
62 Bogousslavsky J, Regli F, Delaloye B, et al.: Loss of psychic self-activation with bithalamic infarction. Acta Neurol Scand 1991; 83:309–316Crossref, Medline, Google Scholar
63 Engelborghs S, Marien MA, Pickut B, et al.: Loss of psychic self-activation after paramedian bithalamic infarction. Stroke 2000; 31:1762–1765Crossref, Medline, Google Scholar
64 Adams RD, Victor M: Principles of Neurology 3rd ed. New York, McGraw-Hill, 1985, p 388Google Scholar
65 Fisher CM: Abulia minor versus agitated behavior. Clin Neurosurg 1983; 31:9–31Medline, Google Scholar
66 Laplane D: La perte d’auto-activation psychique. Rev Neurol (Paris) 1990; 146:397–404Medline, Google Scholar
67 Laplane D, Levasseur M, Pillon B, et al.: Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions: a neuropsychological, magnetic resonance imaging and positron tomography study. Brain 1989; 112:699–725Crossref, Medline, Google Scholar
68 Marin RS: Differential diagnosis and classification of apathy. Am J Psychiatry 1990; 147:22–30Crossref, Medline, Google Scholar
69 Marin RS: Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 1991; 3:243–254Link, Google Scholar
70 Marin RS. Apathy: concept, syndrome, neural mechanisms and treatment. Semin Clin Neuropsychiatry 1996; 1:304–314Medline, Google Scholar
71 Rolls ET: A theory of emotion and consciousness, and its application to understanding the neural basis of emotion in The Cognitive Neurosciences. Edited by Gazzaniga MS. Cambridge, Mass, MIT Press, 1995, pp 1091–1106Google Scholar
72 Apicella P, Ljundberg T, Scarnati E, et al.: Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res 1991; 85:491–500Crossref, Medline, Google Scholar
73 Assal F, Cummings JL: Neuropsychiatric symptoms in the dementias. Curr Opin Neurol 2002; 15:445–450Crossref, Medline, Google Scholar
74 Boyle PA, Malloy PF, Salloway S, et al.: Executive dysfunction and apathy predict functional impairment in Alzheimer disease Am J Geriatr Psychiatry 2003; 11:214–221Google Scholar
75 Ready RE, Ott BR, Grace J, et al.: Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. Am J Geriatr Psychiatry 2003; 11:222–228Crossref, Medline, Google Scholar
76 Marin RS, Butters MA, Mulsant BH, et al.: Apathy and executive function in depressed elderly. Geriatr Psychiatry Neurol 2003; 16:112–116Crossref, Medline, Google Scholar
77 Levy ML, Cummings JL, Fairbanks LA, et al.: Apathy is not depression. J Neuropsychiatry Clin Neurosci 1998; 10:314–319Link, Google Scholar
78 Snowden JS, Bathgate D, Varma A, et al.: Distinct behavioural profiles in frontotemporal dementia and semantic dementia: J Neurol Neurosurg Psychiatry 2001; 70:323–332Google Scholar
79 Aarsland D, Litvan I, Larsen JP: Neuropsychiatric symptoms of patients with progressive supranuclear palsy and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2001; 13:42–49Link, Google Scholar
80 Paulsen JS, Ready RE, Hamilton JM, et al.: Neuropsychiatric aspects of Huntington’s disease. J Neurol Neurosurg Psychiatry 2001; 71:310–314Crossref, Medline, Google Scholar
81 Destée A, Gray F, Parent M, et al.: Comportement compulsif d’allure obsessionnelle et paralysie supranucléaire progressive. Rev Neurol 1990; 146:12–18Medline, Google Scholar
82 Ghika J, Ghika-Schmid F, Fankhauser H, et al.: Bilateral contemporaneous posteroventral pallidotomy for the treatment of Parkinson’s disease: neuropsychological and neurological side effects: report of four cases and review of the literature. J Neurosurg 1999; 91:313–321Crossref, Medline, Google Scholar
83 Swerdlow NR, Koob GF: Dopamine, schizophrenia, mania, and depression: toward a unified hypothesis of cortico-striato-pallido-thalamic function. Behav Brain Sci 1987; 10:197–245Crossref, Google Scholar
84 Levitt JJ, McCarley RW, Dickey CC, et al.: MRI study of caudate nucleus volume and its cognitive correlates in neuroleptic-naive patients with schizotypal personality disorder. Am J Psychiatry 2002; 159:1190–1197Crossref, Medline, Google Scholar
85 Keshavan MS, Rosenberg D, Sweeney JA, et al.: Decreased caudate volume in neuroleptic-naive psychotic patients. Am J Psychiatry 1998; 155:774–778Medline, Google Scholar
86 Corson PW, Nopoulos P, Andreasen NC, et al.: Caudate size in first-episode neuroleptic-naive schizophrenic patients measured using an artificial neural network. Biol Psychiatry 1999; 46:712–720Crossref, Medline, Google Scholar



