Magnetic Resonance Spectroscopy of Memory and Frontal Brain Region in Early Multiple Sclerosis
Abstract
In vivo proton magnetic resonance spectroscopy (1H-MRS) has been used to assess biochemical changes that occur in demyelinating lesions and in normal appearing white matter (NAWM) in multiple sclerosis (MS) patients. N-acetylaspartate (NAA) levels in MS patients may indicate neural viability. In early stages of MS, patients may suffer from slight cognitive impairment. The objective of this study was to investigate memory function in relation to biochemical properties of frontal brain areas of MS patients. Twenty-one patients with relapsing-remitting MS and 21 healthy comparison subjects were examined psychometrically using the Wechsler Memory Scale (WMS) and the Multiple Sclerosis Functional Composite (MSFC) scale, and (1H-MRS) was used to examine frontal deep white matter (left hemisphere) and the frontal cingulate gyrus (Brodmann areas 24/32, bihemispheric). A significant reduction of the NAA/Creatine (Cr) ratio in the frontal cingulate gyrus among the MS patient group was detected when compared to healthy subjects. A significant decrease in the NAA/Cr ratio was also found in volumes of cerebral deep white matter, including plaques, in the MS patients. No NAA/Cr ratio changes were found in NAWM. Differences in MSFC results did not reach statistical significance, but the WMS general memory score showed a significant statistical difference between the patient group and healthy subjects. Regression analysis showed the gray matter NAA/Cr ratio of the frontal cingulate gyrus to be significantly related to distinct memory functions. The authors conclude that (1H-MRS) of gray matter in early stages of MS may be pertinent in the detections of early metabolic disturbances, particularly in subjects with or without minor neurological impairment. Findings suggest a general relationship between the metabolic status of the frontal cortices and memory function.
Multiple sclerosis (MS) is the most common nontraumatic neurological illness among young adults. The disease is characterized by a progressive course and affects multiple areas of the central nervous system (CNS). Following the appearance of neurological symptoms, MS is diagnosed by means of the patient’s history, cerebral spinal fluid (CSF) analysis, magnetic resonance imaging (MRI), and evoked potential recordings.1–3
Even in early stages of MS, patients and healthy comparison subjects may differ in cognitive test performance. Multiple sclerosis patients may demonstrate memory deficits in particular.4–11
Before magnetic resonance spectroscopy was available as a clinical tool, conventional MRI-data (i.e., plaque volume, plaque localization, and brain atrophy) were compared with neuropsychological test scores to correlate structural damage with cognitive function.12–14
Conventional magnetic resonance (MR)-techniques have significant limitations in the diagnosis of MS due to poor specificity for hyperintense changes, particularly in gray matter, and for the detection of changes in otherwise normal appearing white matter. These are two of the main reasons why the correlation between T2 lesion volume and neurological disabilities is only modest in patients with multiple sclerosis.
In vivo proton magnetic resonance spectroscopy (1H-MRS) was developed to assess biochemical changes that occur in many diseases of the brain. The use of 1H-MRS to measure N-acetylaspartate (NAA) offers insight into neuronal processes, revealing a high concentration of NAA disturbances among patients, predominantly in neuronal structures.15–16
A decrease of NAA concentration in brain lesions of MS patients and in normal appearing white matter (NAWM) and its correlation with clinical disabilities has been described in the literature.17–23
Discrepancies concerning frontal activity during memory processes between functional imaging and neuropsychological data may indicate a limitation or insensitivity in behavioral measures. Fletcher and Henson24 suggest that functional imaging data contain important information about the way subjects perform memory tasks.
In the present study, we hypothesized that lower levels of memory in early stages of MS are accompanied by a reduction of NAA in cortical gray matter, which is relevant to cognitive function. Supporting a role of anterior cingulate cortex in a memory network, Fazio et al.25 found bilateral metabolic reductions in the hippocampal formation, thalamus, cingulate gyri, and frontal basal cortex in a positron emission tomography (PET) study. We determined the NAA/Creatine (Cr) ratio in frontal deep white matter and in the cortex of the frontal cingulate gyrus (Brodmann areas 24/32) to be part of the cognition division of the anterior cingulate cortex (including caudal areas 24 and 32, the cingulate motor areas in the cingulate sulcus, and nociceptive cortex). Brodmann areas 24 and 32 are involved in cortical circuits responsible for brain function that requires cognitive processing. Area 24 may involve tasks that require short-term memory but that do not require movement. Tasks requiring target assessment and attention to linguistic and other features of the sensory environment may engage area 32.26,27
METHODS
Patients
Our sample included 21 patients between the ages of 18 and 43 years (mean 33.5; SD=7.5) who were diagnosed according to the criteria of Poser et al.28 and were suffering from definite MS with a relapsing-remitting course for less than 3 years. Only patients without frontal atrophy were included, and the same effect of CSF was deduced in all subjects.
Patients were compared to 21 healthy volunteers who were matched according to right handedness, age, years of education, and sex. Volunteers were between 20 and 45 years of age (mean 31.8; SD=7.4). All subjects signed a written consent form.
Measures
Disability was assessed by using the Expanded Disability Status Scale (EDSS).29
Expanded Disability Status Scale scores of the MS patients did not exceed 2.5 (Median=1.5; Range=0–2.5). No subject had a history of psychiatric disorder.
All subjects were interviewed and tested neuropsychologically using the Wechsler Memory Scale (WMS),30 a standard clinical memory assessment procedure that consists of seven subtests summarizing in a total score. Subjects were also tested using the Multiple Sclerosis Functional Composite (MSFC) scale,31 an outcome measure for MS clinical trials that assesses the dimensions of ambulation/leg function, arm/hand function, and cognition.
Conventional proton MRI was performed with axial T2 weighted fluid attenuated inversion recovery (FLAIR) images (22 slices, slice-thickness 5 mm, matrix 256×256, TR = 4452 msec; TE = 100 msec) and coronal T1 weighted scans (22 slices, slice-thickness 6 mm, matrix 256×256, TR = 560 msec; TE = 14 msec). In vivo proton magnetic resonance spectroscopy examinations of the brain were obtained in a single session using the 1.5 T Philips Gyroscan (Philips Medical Systems, Best, the Netherlands). Axial and coronal images were used to position the volume of interest (VOI) (size 2×2×2 cm3) in the left frontal deep white matter and gray matter of the frontal cingulate gyrus (Brodmann areas 24/32) of both hemispheres. Voxel positioning was done visually according to a template image taken from the atlas of Talairach and Tournoux.32 White matter voxel center coordinates were x=−34, y=10, N=27 (origin: anterior commissure). The voxel center of the gyrus cinguli was positioned to the coordinates x=0, y=27, N=27, and predominantly gray matter structures were within the VOI (Figure 1).
We used a PRESS technique (TR = 2000 msec; TE = 136 msec) with automatic shimming, water suppression by frequency selective adiabatic pulse, time domain filtering by Gaussian, and exponential multiply. Spectra analyses were conducted by measuring peak NAA and Cr. Results were expressed as NAA/Cr ratio, assuming Cr as a stable metabolite.33
Statistics
Comparison between groups was computed using the Chi-Square Test for nominal data and the Mann-Whitney U-Test (two-tailed) for ordinal scaled data. Linear multiple regression analysis (stepwise method) was computed to examine the significance of MRS data an independent variable of memory outcome (SPSS 10.0).
RESULTS
Conventional MRI showed small plaque volumes in all patients. Single plaques in deep white matter of the frontal lobe were found in 33% of the patients. No plaques were found in frontal gray matter or subcortical white matter.
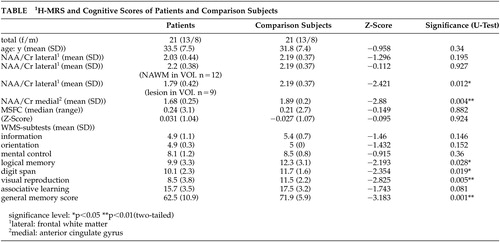
The NAA/Cr ratio of patients in the frontal cingulate gyrus showed a significant reduction (p=0.004) when matched against healthy comparison subjects (Figure 2).
No significant reduction of the NAA/Cr ratio was found in the VOI positioned in the frontal white matter when the whole patient group was compared with healthy subjects (p= 0.195). Dividing patients according to white matter lesions in VOI, the NAA/Cr ratio of the group without lesions (N=12) did not significantly differ from that of healthy subjects (p=0.927). As we anticipated, the group with lesions in white matter VOI (N=9) significantly differed from healthy comparison subjects (p=0.012), as expected.
The multiple sclerosis functional composite scores of patients and healthy subjects did not significantly differ. Multiple sclerosis patients scored significantly lower than healthy comparison subjects in WMS total score (p=0.001). This was also the case for the WMS subtests in logical memory (p=0.028), digit span (p=0.019), and visual reproduction (p=0.005) (Table).
Using NAA/Cr ratios of gray and white matter as independent variables for WMS-scores, linear multiple analysis regression (stepwise) for all subjects showed gray matter NAA/Cr ratio of the frontal cingulate gyrus to be significantly associated with the WMS total score (N=8.105, df=1; p=0.007) (Figure 3); WMS associative learning (N=7.138, df=1; p=0.015); and WMS logical memory (N=6.238, df=1; p=0.016). White matter NAA/Cr ratio was not a significant independent variable in regression analysis for any cognitive test score.
DISCUSSION
Combining 1H-MRS metabolite measurements with cognitive capacities provides new insight into understanding neuropsychological disturbances caused by chronic diseases of the brain such as MS.34–37
In vivo proton magnetic resonance spectroscopy studies of white matter in MS patients38–45 have shown significant correlations between the reduction of NAA and clinical disabilities.17–23 Other studies have found significant associations between cognitive function and the reduction of NAA in white matter.46–48 Recent studies have reported the involvement of gray matter structures in MS patients,49–52 but these studies do not address the correlation between gray matter and cognitive functions.
In our study using in vivo proton magnetic resonance spectroscopy, VOIs were located in the left frontal deep white matter and in the frontal cingulate gyrus (Brodmann areas 24/32), which is an important cortical area for cognitive functions such as attention focusing, short-term memory, and working memory.27,53,54 To minimize the partial volume effect caused by CSF, the voxel was positioned as closely as possible to these Brodmann areas and subjects with atrophy were excluded.
We detected significant reduction of the NAA/Cr ratio in frontal white matter when MS plaques were localized within the VOI. The NAA/Cr ratio in NAWM was not reduced in our patient group, which was in accordance with the low clinical disability.
Also indicating a distinct disturbance of gray matter in MS patients, a significant reduction of NAA/Cr ratio in the frontal cingulate gyrus was found among this patient group when compared to healthy subjects.
Considering the well preserved clinical integrity of the patients in this study, the MS-specific MSFC did not show any significant difference between patients and healthy comparison subjects, which is what we had expected.
We found significant differences in memory abilities, as measured by the WMS general memory score and its subtests: logical memory, digit span, and visual reproduction. These findings are in agreement with the results of a metaanalysis of 36 studies investigating memory deficits in MS-patients.10 This analysis suggests a global memory disorder containing indices for short-term memory, working memory (in particular the simultaneous retention and internalization of information), and long-term memory among MS patients, which differed significantly from that of comparison subjects.
In our sample (patients and healthy comparison subjects), no significant relation between NAA/Cr ratio in white matter and memory scores was found. The NAA/Cr ratio in gray matter of the frontal cingulate gyrus, however, was a significant independent variable in regression analysis, predicting overall memory score and logical and verbal associative memory. These findings may stimulate further debate about the general relation between memory function and NAA-concentration in the cortices.
We conclude that metabolic changes of the cerebral cortex, such as reduction of NAA/Cr ratio in gray matter, may be a sensitive indicator of cognitive function. Therefore, further research should focus on relating additional gray matter metabolic brain mapping with cognitive functions.
ACKNOWLEDGMENTS
This study was presented at the European Federation of Neurological Societies (ENS) Congess, Vienna, October 2002.
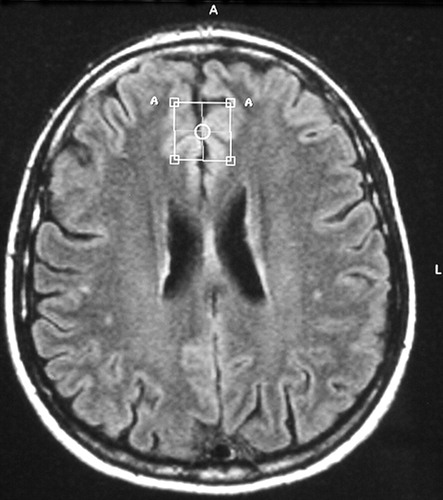
FIGURE 1. Position of the Voxel in Gray Matter of Frontal Cingulate Gyrus Brodmann Areas 32/24 (axial T2 weighted FLAIR image)
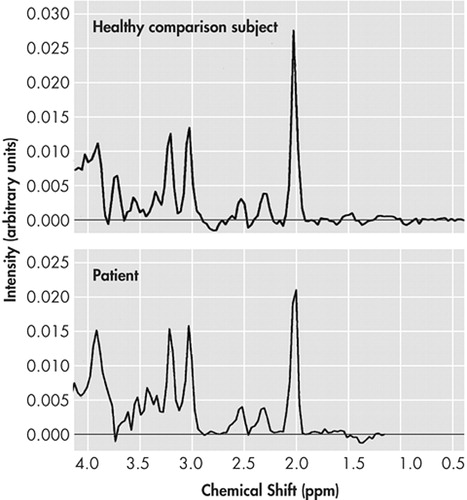
FIGURE 2. 1H-MRSpectrogram of a Healthy Comparison Subject (upper row), 1H-MRSpectrogram of a Patient (lower row) Demonstrating a Reduction of the N-Acetylaspartate-Peak
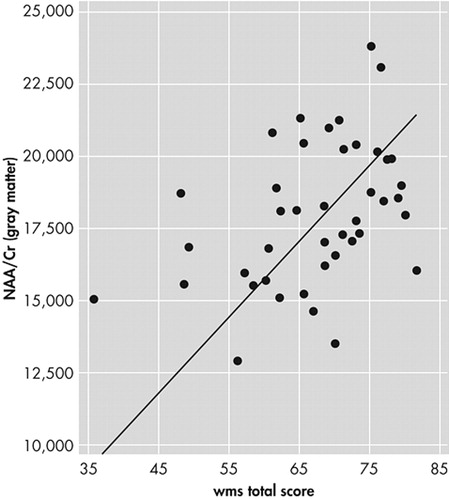
FIGURE 3. Scatterplot With Regression Curve (N=42) of NAA/Cr Ratios in Gray Matter and WMS Total Memory Scores
WMS=Wechsler Memory Scale
 |
1 Farlow MR, Marhand ON, Edwards MK, et al: Multiple sclerosis: magnetic resonance imaging, evoked responses and spinal fluid electrophoresis. Neurol 1986; 36:828–831Crossref, Medline, Google Scholar
2 Paty DW, Oger JJF, Kastrukoff LF, et al: MR in the diagnosis of MS: a prespective study with comparision of clinical evaluation, evoked potentials, oligoclonal banding and CT. Neurol 1988; 38:180–185Crossref, Medline, Google Scholar
3 Staffen W, Trinka E, Ladurner G: Contribution of MRI, multimodal evoked potentials and CSF changes to the diagnosis of multiple sclerosis. Nervenarzt 1993; 64:226–232Medline, Google Scholar
4 Rao SM, Hammeke TA, Mc Quillen MP, et al: Memory disturbances in chronic progressive multiple sclerosis. Arch Neurol 1984; 41:625–631Crossref, Medline, Google Scholar
5 Rao SM: Neuropsychology of multiple sclerosis: A critical review. J Clin Exp Neuropsychol 1986; 8:503–542Crossref, Medline, Google Scholar
6 Van den Burg W, van Zomeren AH, Minderhaud JM, et al: Cognitive impairment in patients with multiple sclerosis and mild physical disability. Arch Neurol 1987; 44:494–501Crossref, Medline, Google Scholar
7 Rao SM, Leo GJ, Unverzagt F: Cognitive dysfunction in multiple sclerosis: frequency, patterns predictions. Neurology 1991; 41:2014–2015Medline, Google Scholar
8 Gilchrist AC, Creed FH: Depression, cognitive impairment and social stress in multiple sclerosis. J Psychosom Res 1994; 38:193–201Crossref, Medline, Google Scholar
9 Amato MP, Ponzaini G, Pracucci G, et al: Cognitive impairment in early onset multiple sclerosis: pattern, predictors, and impact on everyday life in a four year follow up. Arch Neurol 1995; 52:168–172Crossref, Medline, Google Scholar
10 Thornton AE, Raz N: Memory impairment in multiple sclerosis: a quantitative review. Neuropsychol 1997; 11:357–366Crossref, Google Scholar
11 Gaudino EA, Chiaravalloti ND, DeLuca J, et al: A comparison of memory performance in relapsing-remitting, primary progressive and secondary progressive multiple sclerosis. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:32–44Medline, Google Scholar
12 Rao SM, Leo GJ, Haughton VM, et al: Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology 1989; 39:161–166Crossref, Medline, Google Scholar
13 Breteler MB, van Amerongen NM, van Swieten JC, et al: Cognitive correlates of ventricular enlargement and cerebral white matter lesions and magnetic resonance imaging. The rotterdam study. Stroke 1994; 25:1109–1115Crossref, Medline, Google Scholar
14 Comi G, Filippi M, Martinelli V, et al: Brain MRI correlates of cognitive impairment in primary and secondary progressive multiple slerosis. J Neuro Sci 1995; 132:222–227Crossref, Medline, Google Scholar
15 Brand A, Richter-Landsberg C, Leibfritz D: Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 1993; 15:289–298Crossref, Medline, Google Scholar
16 Urenjak J, Willliams SR, Gadian DG, et al: Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 1993; 13:981–989Crossref, Medline, Google Scholar
17 De Stefano N, Matthews PM, Narayanan S, et al: Axonal dysfunction and disability in a relaps of multiple sclerosis: a longitudinal study of a patient. Neurol 1997; 49:1138–1141Crossref, Medline, Google Scholar
18 De Stefano N, Matthews PM, Fu L, et al: Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. results of a longitudinal magnetic resonance spectroscopy study. Brain 1998; 121:1469–1477Crossref, Medline, Google Scholar
19 Arnold DL, Wolinky JS, Matthews PM, et al: The use of magnetic resonance spectroscopy in the evaluation of natural history of multiple sclerosis. J Neurol Neurosurg Psychiatry 1998; 64:S94-S101Google Scholar
20 Brex PA, Gomez-Anson B, Parker GJ, et al: Proton MR spectroscopy in clinically isolated syndromes suggestive of multiple sclerosis. J Neurol Sci 1999; 166:16–22Crossref, Medline, Google Scholar
21 Matthews PM, De Stefano N, Narayanan S, et al: Putting magnetic resonance spectroscopy studies in context: axonal damage and disability in multiple sclerosis. Semin Neurol 1998; 18:327–336Crossref, Medline, Google Scholar
22 Davie DA, Silver NC, Barker GJ, et al: Does extent of axonal loss and demyelinisation from chronic lesions in multiple sclerosis correlate with the clinical subgroup? J Neuro Neurosurg Psychiatry 1999; 67:710–715Crossref, Medline, Google Scholar
23 Lee MA, Blamire AM, Pendlebury S, et al: Axonal injury or loss in the internal capsule and motor impairment in multiple sclerosis. Arch Neurol 2000; 57:65–70Crossref, Medline, Google Scholar
24 Fletcher PC, Henson RNA: Frontal lobes and human memory. insights from functional neuroimaging. Brain 2001; 124:849–881Crossref, Medline, Google Scholar
25 Fazio F, Perani D, Gilardi MC, et al: Metabolic impairment in human amnesia: a PET study of memory networks. J Cereb Blood Flow and Metab 1992; 12:353–358Crossref, Medline, Google Scholar
26 Devinsky O, Morrell MJ, Vogt BA: Contributions of anterior congulate cortex to behaviour. Brain 1995; 118:279–306Crossref, Medline, Google Scholar
27 Cabeza R, Nyberg L: Imaging cognition II: An empirical review of 275 PET and FMRI studies. J Cogn Neurosci 2000; 12:1–47Crossref, Medline, Google Scholar
28 Poser CM, Paty DW, Scheinberger LC, et al: New diagnosis criteria for MS: guidelines for research protocols. Ann Neurol 1983; 13:227Crossref, Medline, Google Scholar
29 Kurtzke J: Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33:1444–1452Crossref, Medline, Google Scholar
30 Wechsler D: A standardised memory scale for clinical use. J Psychol 1945; 87–95Google Scholar
31 Fischer JS, Rudick RA, Cutter GR, et al: The Multiple Sclerosis Functional Composite (MSFC) measure: an integrated approach to MS clinical outcome measurement. Multiple Sclerosis 1999; 5:244–250Crossref, Medline, Google Scholar
32 Talairach J, Tournoux P: Co-planar stereotaxic atlas of the human brain: Three dimensional proportional system. An approach to cerebral imaging. Thieme, Stuttgart: New York 1988Google Scholar
33 Miller BL: A review of chemical issues in 1-H-NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed 1991; 4:47–52Crossref, Medline, Google Scholar
34 Miller DH, Grossman RI, Reingold SC, et al: The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain 1998; 121:3–24Crossref, Medline, Google Scholar
35 Barkhof F, van Waldeveen MA: Characterization of tissue damage in multiple sclerosis by nuclear magnetic resonance. Philos Trans R Soc Lond B Biol Sci 1999; 354:1675–1686Crossref, Medline, Google Scholar
36 Simone IL, Tortorella C, Federico F: The contribution of (1)H-magnetic resonance spectroscopy in defining the pathophysiology of multiple sclerosis. Ital J Neurol Sci 1999; 20: 5 Suppl 241–245Google Scholar
37 van Walderveen MA, Barkhof F, Pouwels PJ, et al: Neuronal damage in T1-hypointense multiple sclerosis lesions demonstrated in vivo using proton magnetic resonance spectroscopy. Ann Neurol 1999; 46: 1 79–87Google Scholar
38 Fu L, Wolfson C, Worsley KJ, et al: Statistics of investigation of multimodal MR imaging data and an application to multiple sclerosis patients. NMR Biomed 1996; 9:339–346Crossref, Medline, Google Scholar
39 Fu L, Matthews N, De Stefano N, et al: Imaging normal-appearing white matter in multiple sclerosis. Brain 1998; 121:103–113Crossref, Medline, Google Scholar
40 Davie CA, Barker GJ, Thompson AJ, et al: 1H magnetic resonance spectroscopy of chronic white matter lesions in normal appearing white matter in multiple sclerosis. J Neurol Neurosurg Psychiatry 1997; 63:736–742Crossref, Medline, Google Scholar
41 Clanet M, Berry I: Magnetic resonance imaging in multiple sclerosis. Curr Opin Neurol 1998; 11:299–303Crossref, Medline, Google Scholar
42 Sarchielli P, Presciutti O, Tarducci R, et al: 1H-MRS in patients with multiple sclerosis undergoing treatment with interferon beta-1a: results of a preliminary study. J Neurol Neurosurg Psychiatry 1998; 64:204–212Crossref, Medline, Google Scholar
43 Sarchielli P, Presciutti O, Pelliccioli GP, et al: Absolute quantification of brain metabolites by proton magnetic resonance spectroscopy in normal appearing white matter of multiple sclerosis patients. Brain 1999; 122:513–521Crossref, Medline, Google Scholar
44 Leary SM, Davie CA, Parker GJ, et al: 1H magnetic resonance spectroscopy of normal apearing white matter in primary progressive multiple sclerosis. J Neurol 1999; 11:1023–1026Crossref, Google Scholar
45 Tourbah A, Stievenart JL, Gout O, et al: Localized magnetic resonance spectroscopy in relapsing remitting versus secondary progressive multiple sclerosis. Neurology 1999; 53:1091–1097Crossref, Medline, Google Scholar
46 Foong J, Rozewicz L, Davie CA, et al: Correlates of executive function in multiple sclerosis: the use of magnetic resonance spectroscopy as an index of focal pathology. J Neurosci Clin Neurosci 1999; 11:45–50Link, Google Scholar
47 Jung RE, Yeo RA, Chiulli SJ, et al: Biochemical markers of cognition: a proton MR spectroscopy of normal human brain. Neuroreport 1999; 10:3327–3331Crossref, Medline, Google Scholar
48 Pan JW, Krupp LB, Elkins LE, Coyle PK: Cognitive dysfunction lateralizes with NAA in multiple sclerosis. Appl Neuropsychol 2001; 8:155–160Crossref, Medline, Google Scholar
49 Kapeller P, McLean, Griffin CM, et al: Peliminary evidence for neuronal damage in cortical grey matter and normal appearing white matter in short duration relapsing-remitting multiple sclerosis: a quantitative MR spectroscopy imaging study. J Neurol 2001; 248:131–138Crossref, Medline, Google Scholar
50 Sharma R, Narayana PA, Wolinsky JS: Gray matter abnormalities in multiple sclerosis: proton magnetic resonance spectroscopic imaging. Mult Scler 2001; 7:221–226Crossref, Medline, Google Scholar
51 McClean MA et al: Metabolite content in cerebral grey and white matter studied with short-echo time 1H-MRSI. In Eighth International Society of Magnetic Resonance in Medicine. April 1–7, 2000, Denver, CO, p 1131Google Scholar
52 Persciutti O, Sarchielli P, Gobbi G:1H MRS study of occipital grey matter of multiple sclerosis patients. In Eighth International Society of Magnetic Resonance in Medicine. April 1–7,2000, Denver, CO, p 627Google Scholar
53 Bush G, Luu P, Posner MI: Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000; 4:215–222Crossref, Medline, Google Scholar
54 Duncan J, Owen AM: Common regions of the human frotal lobe recruited by diverse cognitive demands. Trends Neurosci 2000; 23:475–483Crossref, Medline, Google Scholar



