Neurological Soft Signs as Predictors of Treatment Response to Selective Serotonin Reuptake Inhibitors in Obsessive-Compulsive Disorder
Abstract
Neurological soft-sign abnormalities have been implicated in obsessive-compulsive disorder (OCD). This first comprehensive data analysis evaluated the association between baseline neurological soft signs and treatment response in 117 OCD patients treated with controlled-release fluvoxamine in a double-blind placebo-controlled trial. Total and right-sided soft signs for the responders and the nonresponders did not differ significantly. Left-sided visuospatial soft signs were significantly increased in treatment nonresponders compared to responders. These subtle neurological abnormalities may implicate a potential subgroup of OCD patients with poorer treatment response. This may have treatment implications and therefore serve as a screening tool in OCD.
Obsessive-compulsive disorder (OCD) is a chronic and often disabling disorder. A broad range of evidence exists linking neurological dysfunction to OCD, such as onset of OCD following head trauma,1 encephalitis2 and streptococcal infections.3 There is a high level of comorbidity between OCD and Tourette’s syndrome,4 with overlapping subcortical circuitry mediating both conditions. Abnormalities in the orbital frontal cortex, anterior cingulate, caudate, and thalamus have been demonstrated in OCD patients,5 and modulation of this circuitry with neurosurgical techniques such as cingulotomy, capsulotomy, subcaudate tractotomy, limbic leucotomy and repetitive transcranial magnetic stimulation (rTMS) may provide some clinical improvement in refractory OCD patients.6–8 These biological circuits in the brain are complex, with no one area of neuroanatomical dysfunction pathognomonic in the etiology of OCD.
Different subtypes of OCD may also contribute to neurobiological diversity in the illness. For example, up to 40%–60% of OCD patients may not respond to adequate medication trials with selective serotonin reuptake inhibitors (SSRIs)9 or cognitive behavior treatments (CBT), and this may represent a unique subgroup of patients with treatment resistant OCD. Recent investigations examined different symptom dimensions on the Yale Brown Obsessive-Compulsive Scale (YBOCS) symptom checklist to identify subgroups10 or different symptom clusters11 such as hoarders, who have been shown to have poor clinical response to SSRIs.10 Other subtypes may include those OCD patients with tic disorders who have been shown to respond to augmentation of SRIs with neuroleptics.12
Given evidence for neuroanatomical dysfunction in OCD, questions proposed are what role the neurological exam plays in identifying specific subtypes of OCD patients and whether these subtypes of patients with abnormal exams may have different treatment responses. Neurological soft signs are nonlocalizing deviant performances on motor and/or sensory tests without evidence of focal cerebral dysfunction. Soft signs may reflect small lesions in the brain that manifest themselves by evoking minor neurological abnormalities, as opposed to large lesions, which lead to gross physical and/or computed tomography (CT) or magnetic resonance imaging (MRI) findings. Abnormalities may be in coordination, involuntary movements, sensory areas, and visuospatial areas. There is a documented link between neurological soft signs and psychiatric illness.13–18 The prevalence of neurological soft signs in schizophrenia has been found to range from 50% to 65% versus 5% in healthy comparison subjects.19 Besides their documented presence in adult psychiatric conditions, neurological soft signs may play a role in the neurodevelopment of the brain, as early soft signs have been shown to precede adult-onset schizophrenia18 and anxiety disorders.17
To date, there are few studies of neurological soft signs in OCD, or their relationship to treatment response. A study by Hollander and colleagues13 evaluated 41 medication-free OCD patients for neurological soft signs. Increased neurological soft signs were found in the OCD patients when matched against healthy comparison subjects.13 Significant abnormalities included fine motor coordination, involuntary and mirror movements, and visuospatial function. Visuospatial abnormalities including cube drawing, with an excess of findings on the left side of the body, suggested possible right hemisphere dysfunction in a subgroup of patients with OCD. Of note, these were examined by a neurological soft signs examination. In addition, soft signs correlated with the severity of obsessions. There was also a correlation between abnormalities in visual memory and recognition on neuropsychological testing and total soft signs. Additional analyses of findings in these OCD patients showed that the greater the severity of right-sided soft signs, the worse the treatment outcome.14 Bihari et al.15 evaluated 39 patients with OCD when matched against comparison subjects, and looked at the effect of medication on neurological soft signs. They reported that OCD patients scored significantly higher on neurological soft sign measures than other groups, but no difference was found between medicated and unmedicated patients with OCD.
As yet, there are no data from large samples evaluating the relationship between soft signs at baseline and response to SRI treatment in OCD patients. This is the first comprehensive data analysis evaluating the association between neurological soft signs and treatment response in 117 OCD patients who were treated with fluvoxamine.
METHOD
A 12-week, double-blind, flexible-dose, placebo-controlled, parallel-arm, multicenter trial was conducted to determine the safety and efficacy of a controlled release formulation of fluvoxamine in adult patients with OCD.20 The protocol was approved by institutional review boards at each clinical site. Subjects were randomly assigned to either fluvoxamine or placebo groups following a 1-week placebo washout phase. Dosing began at 100 mg at bedtime which was titrated up weekly (over 6 weeks) as tolerated in 50-mg increments to between 100 mg and 300 mg daily. Adherence during the study was assessed, and those taking <80% or >120% of prescribed dosages on two or more visits were considered nonadherent and discontinued from the study.
Criteria for entry into the study were male or female subjects aged 18 or older, a primary diagnosis of OCD, score≥21 on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS), and score≤16 on the Hamilton Depression Scale (HAM-D). Exclusion criteria were comorbid primary DSM–IV diagnosis (including major depression within the last 6 months), significant risk of suicide, ECT within 90 days prior to the study, history of nonresponse to an adequate trial of an SSRI, need for additional psychotropic medication, unstable medical conditions, pregnancy, and clinically significant abnormal laboratory test results. In addition, women of childbearing potential who were not using a medically acceptable method of contraception were excluded. Prior to randomization, all subjects received a complete description of the study and gave written informed consent.
All patients had a structured neurological soft signs examination at baseline, as described by Hollander et al.13 This was found to have very good interrater reliability ranging from 0.58 to 0.95.13 The neurological soft sign examination is a 20-item scale, divided into four categories: coordination, involuntary movements, sensory, and visuospatial function. All subjects were evaluated by psychiatrists who were extensively trained by Dr. Hollander on this neurological soft signs examination, and reviewed videotapes on the neurological soft signs examination, reaching levels of expertise prior to the onset of the trial.
Coordination was assessed for accuracy in the following areas: finger to finger, finger to nose, heel to shin, finger to thumb, rapid alternating movements, mirror movements, hopping, and toe and heel walking. To evaluate speech, two tongue twisters were done by the patients. Involuntary movement testing included standing in the Romberg position, with the first 20 seconds assessing station and motor persistence. For the next 20 seconds, assessment of athetosis, chorea, tremor (resting and intention), and abnormal posturing was conducted. Sensory function included testing for astereognosis, agraphesthesia, position sense, and direction of cutaneous kinesthesia. Visuospatial station was assessed using the face-hand test, right-left confusion on self and examiner, and cube drawing. The face-hand test involved touching the face and hands of the patient simultaneously, in different asymmetric combinations, to assess for sensation deficits. In testing right-left confusion, right and left sided body parts on the patient and examiner were tested for misidentification. On the cube drawing, abnormalities in drawing a three dimensional cube were assessed.
Patients were instructed to perform tasks in these categorical areas in which performance was observed and rated. Each of the 20 items was rated as Yes (=1) or No (=0), indicating presence or absence of soft signs. Eighteen of the items with the exception of tongue twisters and cube drawing were scored separately for the right and left hand. In addition to the individual items, summary measures were the total number of soft signs, right versus left sided soft signs, and categorical totals.
Severity of OCD symptoms and response to medication was assessed by Y-BOCS, Clinical Global Impression Severity of Illness (CGI-S) scale, and Clinical Global Impression Improvement (CGI-I) scale. During the treatment phase, subjects were assessed biweekly for compliance, Clinical Global Impression (CGI) and Y-BOCS scores.
Treatment response was defined as CGI-I ratings of ldquo;1, very much improved” or “2, much improved.” Other responder measures determined from post hoc analyses included Y-BOCS decreases of 25%, and remission status of Y-BOCS≤16 at endpoint.20
Based on previous results, we hypothesized that severity of total baseline neurological soft signs, left-sided visuospatial abnormalities, and total right-sided soft signs would not be associated with treatment response in the 117 patients who received fluvoxamine CR, and we will reject each of the hypotheses at 0.05 level of significance respectively. Since all three soft signs predictors were totals of specific tasks, the significant conclusions for three hypothesis tests will be simultaneously held at the 0.05/3=0.0167 level of significance, by the Bonferroni inequality.
RESULTS
By the criterion of “very much improved,” rated 1, or “much improved,” rated 2, on the CGI-I, there were 51 responders and 66 nonresponders. Baseline neurological soft signs data were not available for 1 subject in each outcome group. Total neurological soft signs for the responders (mean=1.76 [SD=2.55]) and the nonresponders (mean=1.62 [SD=2.18]) did not differ significantly (t=0.33, df=113, p=0.74). Total right-sided soft signs for the responders (mean=0.80 [SD=1.28]) and the nonresponders (mean=0.74, [SD=1.14]) also did not differ significantly (t=0.27, df=113, p=0.79). In contrast, left-sided visuospatial soft signs for the responders (mean=0.10 [SD=0.36]) and the nonresponders (mean=0.32 [SD=0.53]) did significantly differ (t=2.66, df=113, p=0.009) by the Bonferroni inequality criterion (Figure 1).
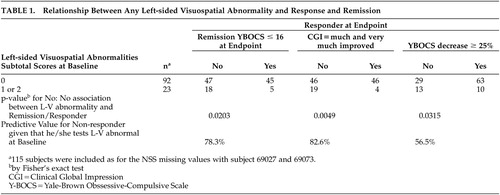
It is clear from the small means that most subjects had no left-sided visuospatial soft signs. When left-sided visuospatial soft signs were dichotomized as none versus one or more, they significantly predicted improvement by two other criteria. Significantly more patients who met remission status (Y-BOCS≤16 at endpoint) had no left-sided soft signs (49%, 45 of 92) than had soft signs (22%, 5 of 23), (p=0.02 by Fisher’s exact test). In addition, significantly more patients who met responder status (Y-BOCS decrease≥25% at endpoint) had no left-sided soft signs (68%, 63 of 92) than had soft signs (43%, 10 of 23), (p=0.03 by Fisher’s exact test). Patients who had any left-sided visuospatial abnormalities were significantly more likely to be nonresponders and nonremitters (Table 1).
DISCUSSION
This study is unique in that it provides the first large sample size evaluating the relationship between neurological soft signs at baseline and response to SSRI treatment in OCD patients. The above finding of more left-sided visuospatial neurological soft sign abnormalities in SSRI nonresponders than responders may implicate subtle neurological abnormalities as predictors of pharmacological treatment response in OCD. This adds to findings from our previous study13 in which OCD patients had significantly more visuospatial soft sign abnormalities than a comparison group, and further supports the role of subtle neurological dysfunction in OCD.
Neuroimaging and other neuroanatomical investigations have pointed to dysfunction in orbitofrontal-subcortical circuitry in OCD patients.5 Neuroanatomical localization in OCD is important in further understanding the etiology of the illness and identifying potential areas for specific treatments such as neurosurgical interventions. Right-hemispheric involvement has been suggested in OCD through prior clinical protocols,13 imaging studies,21–23 and neurosurgical techniques.24 Examples include significant increases in right-sided caudate head volumes on MRI in OCD patients when matched against comparison subjects,21 decreased right caudate metabolism in OCD fluoxetine or CBT responders compared with nonresponders,22 and OCD severity significantly correlated with right orbital frontal cortex metabolism.23 An analysis after thermocapsulotomy or gamma knife capulotomy for OCD identified a lesion volume within the right-sided anterior limb of the internal capsule in all successfully treated patients.24 Gross visuospatial dysfunction is often localized to the nondominant or right parietal lobe. Our subtle left-sided (right brain) visuospatial findings in nonresponders is an addition to the literature on neuroanatomical localization in OCD and may be beneficial early on as a screening tool in identifying subtle neurological abnormalities.
Although we found significantly more left-sided neurological soft signs in nonresponders to SSRIs, it should be noted that there was a low frequency of these events and even minor changes could have impacted on these findings. Another potential limitation to this study was the OCD population tested, as refractory OCD patients or those patients with other primary Axis I disorders were excluded. Therefore, this homogeneous population may not reflect the overall OCD population where comorbid primary conditions or refractory illness are often prevalent. Different soft sign distributions may be prevalent in more heterogenous samples, as reflected by our earlier study of 41 OCD patients, where greater severity of right-sided soft signs was associated with worse treatment outcome.14 In addition, coexistent neurological disorders were not studied in our study and could also have implications on treatment response and distribution of soft signs. Future trials should examine refractory patients with higher comorbidity rates as well as concurrent neurological illness.
OCD is an often difficult to treat condition with most patients not achieving remission, even when responding to SRIs. It is, therefore, important to determine what other mechanisms are in place that have an effect on treatment response. It is possible that a subtype of OCD patients may have right brain impairment, as seen in subtle soft-sign abnormalities in visuospatial function and less response to SSRI treatment. Neurological soft signs may be somewhat related to tic-related problems as up to 40% of patients with Tourette’s syndrome have OCD. Neuroleptic augmentation has been shown to be effective in OCD patients with comorbid Tourette’s syndrome.12 Perhaps, OCD patients with right brain dysfunction may also preferentially respond to neuroleptic augmentation.
Future studies might utilize concurrent neuroimaging measures such as positron emission tomography (PET) or functional MRI with examination of neurological soft signs for more specific localization. Evaluation of family members of OCD patients should be undertaken to determine the familial nature of neurological soft signs and its association with treatment response. Evaluation of the relationship between soft signs in OCD and comorbidity is also suggested. Ultimately a screening tool for soft signs might help guide treatment selection or suggest alternate procedures for treatment in OCD patients.
ACKNOWLEDGMENTS
This study was funded by a grant from Solvay Pharmaceuticals, Inc, & NIDA T32 DA 07135-22 Training Grant (AK).
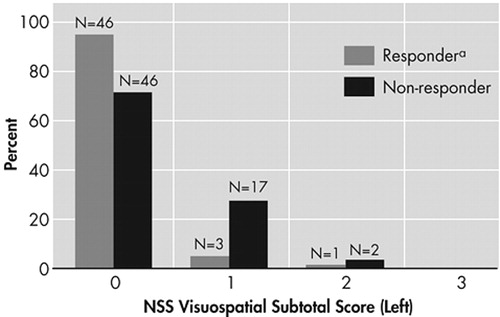
FIGURE 1. Percent-Distribution of NSS Visuospatial Subtotal Score (Left) in 50 Responders and 65 Nonresponders of Fluvoxamine CR Group
aresponder: Clinical Global Impression = much or very much improved at endpoint
 |
1 McKeon J, McGuffin P, Robinson P: Obsessive-compulsive neurosis following head injury: a report of 4 cases. Br J Psychiatry 1984; 144:190–192Crossref, Medline, Google Scholar
2 Schilder P: The organic background of obsessions and compulsions. Am J Psychiatry 1938; 94:1397Crossref, Google Scholar
3 Swedo SE, Rappaport JL, Cheslow DL, et al: High prevalence of obsessive-compulsive symptoms in patients with syndenham’s chorea. Am J Psychiatry 1989; 146:246–249Crossref, Medline, Google Scholar
4 Pauls DL, Towbin KE, Leckman JF et al: Gilles de la Tourette’s syndrome and obsessive-compulsive disorder. evidence supporting a genetic relationship. Arch Gen Psychiatry 1986; 43:1180–112Crossref, Medline, Google Scholar
5 Saxena S, Rauch SL: Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psych Clin North Am 2000; 23:563–586Crossref, Medline, Google Scholar
6 Baer L, Rauch SK, Ballantine T, et al: Cingulotomy for intractable obsessive compulsive disorder. Arch Gen Psychiatry 1995; 43:384–392Crossref, Google Scholar
7 Greenberg BD, George MS, Martin DJ, et al: Effect of prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a preliminary study. Am J Psychiatry 1997; 154:867–869Crossref, Medline, Google Scholar
8 Greenberg BD, Murphy DL, Rasmussen SA: Neuroanatomically based approaches to obsessive-compulsive disorder. Psych Clin North Am 2000; 23:671–686Crossref, Medline, Google Scholar
9 Goodman WK, McDougle CJ, Barr LC, et al: Biological approaches to treatment-resistant OCD. J Clin Psychiatry 1993; 54:16–26Medline, Google Scholar
10 Mataix-Cols D, Rauch SL, Manzo PA, et al: Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatry 1999; 156:1409–1416Medline, Google Scholar
11 Leckman JF, Zhang H, Alsobrook JP, et al: Symptom dimensions in obsessive-compulsive disorder: toward quantitative phenotypes. Am J Med Genetics 2001; 105:28–30Crossref, Medline, Google Scholar
12 McDougle CJ, Goodman WK, Price LH: Dopamine antagonists in tic-related and psychotic spectrum obsessive-compulsive disorder. J Clin Psychiatry 1994, 53(suppl 3):24-31Google Scholar
13 Hollander E, Schiffman E, Cohen B, et al: Signs of central nervous system dysfunction in obsessive-compulsive disorder. Arch Gen Psychiatry 1990; 47:27–32Crossref, Medline, Google Scholar
14 Hollander E, DeCaria CM, Saoud JB, et al: Reply to neurologic soft signs in obsessive-compulsive disorder. Arch Gen Psychiatry 1991; 48:278–279Crossref, Medline, Google Scholar
15 Bihari K, Pato MT, Hill JL, et al: Neurologic soft signs in obsessive-compulsive disorder. Arch Gen Psych 1991; 46 278-79Google Scholar
16 Dazzan P, Murray DM: Neurological soft signs in first-episode psychosis: a systematic review. Br J Psychiatry 2002; 43(Sept suppl):50-57Google Scholar
17 Shaffer D, Schonfeld IS, O’Connor PA, et al: Neurological soft signs and their relationship to psychiatric disorder and intelligence in childhood and adolescence. Arch Gen Psychiatry 1985; 42:342–351Crossref, Medline, Google Scholar
18 Leask SJ, Done DJ, Crow TJ: Adult psychosis, common childhood infections and neurological soft signs in a national birth cohort. Br J Psychiatry. 2002 Nov; 181:387-392Google Scholar
19 Heinrichs DW, Buchanan RW: Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry 1988; 145:11–18Crossref, Medline, Google Scholar
20 Hollander E, Koran LM, Goodman W, et al: A double-blind, placebo-controlled study of the efficacy and safety of controlled release fluvoxamine in patients with obsessive compulsive disorder. J Clin Psychiatry 2003; 64:640–647Crossref, Medline, Google Scholar
21 Scarone S, Colombo C, Livian S, et al: Increased right caudate nucleus size in obsessive-compulsive disorder: Detection with magnetic resonance imaging, Psychiatry Res 1992; 45115-121Google Scholar
22 Baxter LR, Jr, Schwartz JM, Bergman KS, et al: Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49:681–689Crossref, Medline, Google Scholar
23 Swedo SE, Schapiro MG, Grady CL, et al: Cerebral glucose metabolism in childhood onset obsessive-compulsive disorder. Arch Gen Psychiatry 1989; 46:518–523Crossref, Medline, Google Scholar
24 Lippitz BE, Mindus P, Meyerson BA, et al: Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: revelance of the right hemisphere. Neurosurgery 1999; 44:452–458; discussion 458-60Crossref, Medline, Google Scholar



