High–Resolution Brain SPECT Imaging and Eye Movement Desensitization and Reprocessing in Police Officers With PTSD
Abstract
Eye movement desensitization and reprocessing (EMDR) has been shown to be an effective treatment for posttraumatic stress disorder (PTSD). In this study, the authors evaluated the effectiveness and physiological effects of EMDR in police officers involved with on-duty shootings and who had PTSD. Six police officers involved with on-duty shootings and subsequent delayed-onset PTSD were evaluated with standard measures, the Posttraumatic Stress Diagnostic Scale, and high-resolution brain single photon emission computed tomography (SPECT) imaging before and after treatment. All police officers showed clinical improvement and marked reductions in the Posttraumatic Stress Diagnostic Scale Score (PDS). In addition, there were decreases in the left and right occipital lobe, left parietal lobe, and right precentral frontal lobe as well as significant increased perfusion in the left inferior frontal gyrus. In our study EMDR was an effective treatment for PTSD in this police officer group, showing both clinical and brain imaging changes.
Individuals in law enforcement are at a much greater risk of experiencing traumatic events than are average citizens.1 Over the course of their careers, many police officers experience traumatic events that result in posttraumatic stress disorder (PTSD), or they endure traumatic experiences that accumulate and manifest in delayed-onset PTSD upon reaching some threshold. Our clinical experience finds that, without complete recovery, these officers experience diminished ability to manage the chronic stressors and dangers inherent in their jobs.2
Imaging studies have identified several brain regions implicated in PTSD. In case-controlled studies using single photon emission computed tomography (SPECT), Shin et al.3 and Sachinvala et al.4 found increased activity in the limbic regions, particularly in the posterior cingulate gyrus, amygdaloid complex, and right basal ganglia. Also using SPECT, Zubieta et al.5 found significant increases in the blood flow to the medial prefrontal cortex in PTSD patients but not in the comparison groups, which correlated at trend levels with psychophysical measures of stress response. Activation studies conducted by Berthier et al.,6 using SPECT, Benkelfat et al.,7 using positron emission tomography (PET), and Liberzon et al.,8 using SPECT, each found PTSD to be associated with increased paralimbic activity. Using functional magnetic resonance imaging (fMRI), Rauch et al.9 found PTSD patients to exhibit exaggerated amygdala responses to masked-fearful versus masked-happy faces.
A number of studies have shown eye movement desensitization and reprocessing (EMDR) therapy to be an effective treatment for PTSD.10–18 Additionally, brain SPECT imaging has been used to show before- and aftertreatment effects. In a case study design, Levin et al. found activations in the anterior cingulate gyrus and left frontal lobe in four of six patients after three sessions of EMDR.19 Using high-resolution brain SPECT imaging to examine the effects of EMDR on both clinical outcomes and regional cerebral blood flow in six police officers with delayed-onset PTSD, the research presented in this study goes beyond the case study design.
The precise causes of EMDR’s effectiveness remain unknown. A number of studies have found it to be the therapeutic equivalent of exposure therapy,20,21 while other studies equate its effects to cognitive behavior therapy.22 However, Levin et al. argue that therapeutic components specific to EMDR activate a cognitive network that helps patients differentiate real threats from imagined ones.19 We hypothesized that subjects would experience significantly improved clinical outcomes and that these improvements would be reflected in some functional pattern of activity in their respective post-EMDR brain SPECT scans.
METHOD
Subject Selection
All subjects in this study sought treatment for duty-induced PTSD (N=6). Five subjects were right-handed, and one was left-handed. Three subjects had been in talk therapy for duty-induced trauma prior to the study. Among these three, two presented with severe PTSD symptoms and one with moderate symptoms. The remaining 3 subjects had never been in therapy for duty-induced trauma. Among them, two presented with moderate symptoms and one with severe symptoms. There were three criteria for inclusion. First, because of the high incidence of PTSD among officers involved in shootings,1 only subjects who had fired a weapon in the line of duty were included. A second requirement was that a third-party clinician had to confirm clinical diagnoses of PTSD using DSM–IV criteria. Third, the score on the Foa Posttraumatic Stress Diagnostic Scale (PDS), a 49-item self-report instrument designed to aid in the detection and diagnosis of PTSD,23 must be in the moderate to severe range (scores greater than 21 on a scale of 0–51). Because the study included pharmacologically sensitive functional brain imaging, subjects who began the study on medications were required to remain on the same dosage throughout. Five of the six subjects were on no medications, and one remained on an antidepressant only (Celexa 20 mg.). The mean age of subjects was 38.6 years (SD=7.69 years; minimum=31, maximum=50), and the mean level of education was 2 years of college. Four of six subjects had PTSD symptoms that emerged between 42 and 63 months after they were involved in the shooting. Two subjects experienced a shooting within 3 months of the onset of PTSD. A range of symptom severity was sought across our study population to increase the generalizability of our results.
Treatment Protocol
Upon seeking treatment from a therapist contracted with a police department, patients gave a personal history and submitted to an unstructured clinical diagnostic interview to establish a diagnosis of PTSD; likewise, they completed the PDS scale at this time. Potential subjects who met the preliminary criteria of a clinical PTSD diagnosis and who had a PDS score greater than 21 were given the opportunity to participate in the study, whereupon they were given specific details and signed informed consent. All interviews were conducted by the same therapist, who is level II certified by the EMDR International Association.
The treatment format for all subjects proceeded in the following three phases. Phase 1 was clinical, wherein histories were taken for each subject. Subjects were taught coping and “containment” techniques, how to identify and develop support networks,24 and how to log their trauma-related memories—a necessary precondition for EMDR. At the end of Phase 1 we acquired the first (pre-EMDR) brain SPECT scans.
In Phase 2, subjects began EMDR. For all EMDR sessions in this study, we used a TheraTapper, which gave bilateral stimulation in the subjects’ palms and fingers, thus allowing them to reexperience traumatic scenes with their eyes closed. Eye movement has been shown to be effective among law enforcement subjects.18 In our clinical experience, however, police officers have complained about being distracted by the eye movement element in EMDR more than other patient populations. We thus chose to use an eyes-closed mode of EMDR to reduce the possibility of distraction. Although there are no published data on the efficacy of bilateral stimulation in lieu of eye movements, this mode of EMDR has been used clinically for over a decade and is approved by the EMDR International Association.24 In all cases it was observed that unsolicited REM-like activity occurred. EMDR sessions typically ran two to 3 hours in length and were conducted three to 4 weeks apart, resulting in a great deal of mental and emotional fatigue among subjects. Because of the nature of the subjects’ jobs in law enforcement and their associated risks, the frequency and duration of sessions in this study were dictated by each subject’s individual recuperation time. The mean number of EMDR sessions was 3.83 (SD=2.41), and the mean number of EMDR hours was 10.25 (SD 4.84).
Before the final (post-EMDR) PDS scores and SPECT images were acquired, a minimum of 3 weeks was allowed to lapse after the final EMDR session for two reasons. First, it was estimated that this would allow the brain to recover from any EMDR-induced mental fatigue. Second, it would allow some period for subjects’ brain function to become regularized and also for any short-term functional effects to dissipate. Phase 3 constituted a “reconciliation phase” of treatment, focusing on the rescripting of relational patterns that might not have been corrected once subjects became detraumatized.
Study Design, Data Collection, and Statistical Analysis
Two sets of data were collected for analysis in this study: brain SPECT images and PDS scale measurements. Scan data for each of 6 subjects were first collected immediately prior to Phase 2 treatment, and again 3 weeks after the completion of Phase 2, for a total of 12 scans (six pre- and six post-EMDR). PDS scores were acquired prior to Phase 1, and again 3 weeks after the completion of Phase 2, for a total of 12 PDS scores (six pre- and six post-EMDR). Phase 3 began after the final PDS and SPECT were performed. The study design was a simple single-group pre-to-posttest comparison using t tests for both sets of data, as described below.
Brain SPECT Protocol and Image Acquisition
The brain SPECT studies were performed in the following manner. Each subject was placed in a dimly lit, quiet room. Intravenous access was obtained via small-gauge butterfly. Subjects remained quiet for several minutes with open eyes to allow acclimation to their environment. Images were acquired while subjects performed a clinically standardized concentration task, the Connors Continuous Performance Test (CPT), a 15-minute computerized test of attention. We chose to scan the police officers during a concentration task (as opposed to other studies that scanned subjects while reexperiencing the traumatic event) to more accurately simulate their day-to-day functioning. CPT scores were not recorded.
For both the pre- and post-EMDR brain images, subjects began the Connors CPT, and at 3 minutes they were injected with a 3 ml bolus containing 22 mCi of technetium technetium-99m exametazime (commercially available as Ceretec®). Tomographic brain imaging was then performed approximately 45 minutes later using a high-resolution Picker Prism 3000 gamma camera with fan beam collimators. Data were acquired in 128-by-128 matrices. One hundred twenty images with 3° of separation spanning 360° rotation were obtained. The data were prefiltered using a low-pass filter with a high cutoff. Attenuation correction was performed using linear methods. Coronal, sagittal, and transaxial tomographs were parallel to the orbitomeatal line.
Statistical Analyses
Statistical analyses of pre- and post- SPECT images were performed using Statistical Parametric Mapping ’99 (SPM99).25 SPM99 performs voxel-by-voxel analyses using general linear methods, and it requires that all SPECT scans be spatially preprocessed. Thus each pre-EMDR scan was coregistered to its respective post-EMDR scan using the realign function, which created a 12-parameter realignment matrix for each of 12 pairs of pre- and post-EMDR scans. Each pair was then visually inspected for coregistration errors. All 12 scans from both conditions were then, using the Talairach map,26 normalized to a single standardized anatomical space using sinc interpolation and an 8-by-8-by-8 voxel kernel. Finally, all images were smoothed to 7 mm3 using a Gaussian kernel. Smoothing the data results in voxel clusters that better conform to the requirements of Gaussian field theory, which allows us to make more reasonable statistical inferences about our data.27 Smoothing also tends to minimize smaller, more statistically aberrant results.
We performed a global paired t test, which tests the hypotheses,
H1: μ ≠ 0; H0: μ = 0,
at each voxel, where μ is the mean difference in the frequency of photon emission from t1 to t2 . Global perfusion values were proportionally rescaled to 50/ml/dl/min. Threshold masking was used to ensure that only voxels that represent brain activity were included in the analyses, and the threshold for inclusion was set at 80% of the mean global voxel value. The initial significance threshold was set at p=<.001.
Statistical comparisons were made of pre- and post-EMDR PDS scores using pairwise t tests in SAS v8.2.
RESULTS
On the Foa PDS scale, our clinical outcome measure, there was a highly significant decrease from pre- to post-EMDR treatment scores, falling from a pre-EMDR mean of 43.2 ([SD=12.3] minimum=14, maximum=47—severe PTSD range) to a post- mean of 5.2 ([SD=1.9] minimum=1, maximum=6—mild to no PTSD symptoms). None of the six subjects had a post-EMDR PDS score above 6, indicating nearly complete alleviation of clinical PTSD symptoms. A standard pooled t test requires that the variance of the two groups being compared be distributed similarly. As the distribution of scores was significantly different from pre- to post-EMDR (folded F=40.21, p=0.001), a Satterthwaite adjustment was made for comparing groups with unequal variances (t= −5.34, p=0.003).
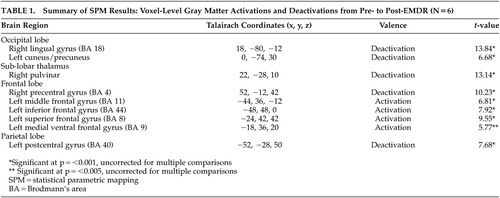
Our SPM analysis yielded five significant deactivations and three activations at the voxel level. All results assume a height threshold uncorrected for multiple comparisons unless otherwise noted. These results are summarized in Table 1.
There were significant deactivations in the right thalamus, the precentral gyrus of the right frontal lobe, and the postcentral gyrus of the left parietal lobe, and there were bilateral deactivations in the occipital lobes (see Figure 1). Likewise, there were significant activations in the left frontal lobe, notably in the middle frontal, inferior frontal, and superior frontal gyri. Most notable is the 320-voxel cluster activation in the inferior frontal gyrus (p =0.001, corrected for multiple comparisons). (Figure 1.)
DISCUSSION
The significant decreases in PDS scores were consistent with the clinical improvement seen in the police officers and the reported effectiveness of EMDR in PTSD. Likewise, our SPM analyses found significant functional differences in brain activity from pre- to post-EMDR imaging. EMDR and the procedures involved with this treatment had both a positive clinical effect and a possible role in changing brain function.
Prior research has found activations in PTSD subjects in the posterior3,4 and anterior cingulate gyrus5,6 and the amygdala;3–6,8 thus, we might have expected post-EMDR deactivations in these areas. Instead we saw deactivations in three areas found by Gundel et al. to be associated with image-induced grief28: the left cuneus, which has been suggested to process motor imagery;29,30 the right lingual gyrus, which has been implicated in processing motor imagery30 and in judging emotionally evocative stimuli;31 and Brodmann’s area (BA) 4 of the right precentral gyrus. Deactivations in these areas may be relieving trauma-induced grief.
We saw further deactivations in the left parietal lobe at BA 40, known to be an association area, and in the right pulvinar. Although the primary function of the pulvinar is unclear, it is thought to be an associative thalamic nucleus that helps regulate cortical circuitry.32 The combination of these two deactivations along with those implicated in grief may constitute the diminution of a network of traumatic memories.
In addition to right hemisphere decreases, our SPM analyses showed three left prefrontal cortical activations, in Brodmann areas 8, 11, and 44. Recent research has found PTSD subjects to have low activity in BAs 8 and 44,33,34 and thus significant activations in these areas appear to be directly correlated with subjects’ improved PDS scores. Further studies have found that depression is linked to low activity in the dorsolateral prefrontal cortex (DLPFC)39–40 and that increased activity in this area is associated with improvements in clinical outcomes.38 Our finding of activation in BA 11 of the DLPFC is consistent with this research and with the reported effects of EMDR on symptoms of depression.19
Many studies having to do with both mood and cognition find that the DLPFC activates reciprocally with the medial ventral prefrontal cortex (MVPFC).36–40 Studies by Goel and Dolan have found that subjects engaged in emotional reasoning show activation in the MVPFC and deactivation in the DLPFC and that this pattern reverses when subjects are engaged in logic.37,40 Although we saw no significant activity at p=0.001, we did see MVPFC activation at p=0.005 (BA 9, Talairach coordinates x=–18, y=36, z=20). This finding may indicate that EMDR brings both modes of cognition to bear on PTSD recovery.
There are two significant limitations to this study. First, our sample contained a left-handed subject, a consequence of the nature by which our sample was recruited. Second, our design was a nonexperimental pretest/posttest comparison. Without a control group this study was subject to validity threats such as history and maturation effects, and thus we cannot make scientific claims about the effects of EMDR on clinical outcomes. Instead we must extrapolate based on the clinical success of prior experimental studies,13–19 and we must further extend this reasoning to the differences we found in post-EMDR brain function. Additional imaging studies using more robust experimental designs are needed to confirm these results.
In summary, our findings of the specific increases and decreases seen on SPECT are consistent with many etiological improvements, including depression41–43 and general affective disorders.44–48 These analyses find EMDR to be associated with significant changes in brain function as measured by SPECT, and that the emergent post-EMDR pattern of brain activity is consistent with changes that may be mitigating PTSD.
ACKNOWLEDGMENTS
Partial funding for SPECT scans was provided by the Eye Movement Desensitization and Reprocessing International Association and Amen Clinics, Inc.
The authors thank Jill Prunella, Research Associate, for her contributions.
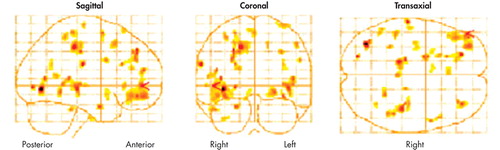
FIGURE 1. Glass Brain Representations of Activations in Post-EMDR Brains at p=<0.005a
Posterior Anterior Right Left Right
An SPM glass brain representation of activations at p=<0.005 for each voxel. Degrees of freedom for individual voxels = 1, 5; number of voxels was 217,449. p=<0.001.
 |
1 Rivard JM, Dietz P, Martell D, et al: Acute disassociative responses in law enforcement officers involved in critical shooting incidents: the clinical and forensic implications. J Forensic Sci 2002; 47:1093–1100Medline, Google Scholar
2 Abrams KM, Robinson GE: Occupational effects of stalking. Can J Psychiatry 2002; 47:468–472Crossref, Medline, Google Scholar
3 Shin LM, Orr SP, Carson MA, et al: Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 2004; 61:168–176Crossref, Medline, Google Scholar
4 Sachinvala N, Kling A, Suffin S, et al: Increased regional cerebral perfusion by 99mTc hexamethyl propylene amine oxime single photon emission computed tomography in post-traumatic stress disorder. Curr Psychiatry Rep 2002; 4:254–263Crossref, Medline, Google Scholar
5 Zubieta JK, Chinitz JA, Lombardi U, et al: Medial frontal cortex involvement in PTSD symptoms: a SPECT study. J Psychiatr Res 1999; 33:259–264Crossref, Medline, Google Scholar
6 Berthier ML, Posada A, Puentes C: Dissociative flashbacks after right frontal injury in a Vietnam veteran with combat-related posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci 2001; 13(1):101–105Link, Google Scholar
7 Benkelfat C, Bradwejn J, Meyer E, et al: Functional neuroanatomy of CCK4-induced anxiety in normal healthy volunteers. Am J Psychiatry 1995; 152:1180–1184Crossref, Medline, Google Scholar
8 Liberzon I, Taylor SF, Amdur R, et al: Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry 1999; 45:817–826Crossref, Medline, Google Scholar
9 Rauch SL, Whalen PJ, Shin LM, et al: Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 2000; 47:769–776Crossref, Medline, Google Scholar
10 Shapiro F: EMDR 12 years after its introduction: past and future research. J Clin Psychol 2002; 58:1–22Crossref, Medline, Google Scholar
11 Carlson J, Chemtob CM, Rusnak K, et al: Eye movement desensitization and reprocessing (EMDR): treatment for combat-related post-traumatic stress disorder. J Trauma Stress 1998; 11:3–24Crossref, Medline, Google Scholar
12 Edmond T, Rubin A, Wambach K: The effectiveness of EMDR with adult female survivors of childhood sexual abuse. Soc Work Res 1999; 23:103–116Crossref, Google Scholar
13 Ironson GI, Freund B, Strauss JL, et al: Comparison of two treatments for traumatic stress: A community-based study of emdr and prolonged exposure. J Clin Psychol 2002; 58:113–128Crossref, Medline, Google Scholar
14 Lee C, Gavriel H, Drummond P, et al: Treatment of post-traumatic stress disorder: a comparison of stress inoculation training with prolonged exposure and eye movement desensitization and reprocessing. J Clin Psychol 2002; 58:1071–1089Crossref, Medline, Google Scholar
15 Marcus S, Marquis P, Sakai C: Controlled study of treatment of PTSD using EMDR in an HMO setting. Psychotherapy 1997; 34:307–315Crossref, Google Scholar
16 Power KG, McGoldrick T, Brown K, et al: A controlled comparison of eye movement desensitization and reprocessing versus exposure plus cognitive restructuring, versus waiting list in the treatment of post-traumatic stress disorder. J Clin Psychol and Psychotherapy 2002; 9:299–318Crossref, Google Scholar
17 Soberman GB, Greenwald R, Rule DL: A controlled study of eye movement desensitization and reprocessing (EMDR) for boys with conduct problems. J Aggression, Maltreatment, and Trauma 2002; 6:217–236Crossref, Google Scholar
18 Wilson SA, Tinker RH, Becker LA, et al: Stress management with law enforcement personnel: a controlled outcome study of EMDR versus a traditional stress management program. Int J Stress Mgt 2001; 8:179–200Crossref, Google Scholar
19 Levin P, Lazrove S, van der Kolk B: What psychological testing and neuroimaging tell us about the treatment of posttraumatic stress disorder by eye movement desensitization and reprocessing. J Anxiety Disord 1999; 13:159–172Crossref, Medline, Google Scholar
20 Davidson R, Parker KCH: Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol 2001; 69:305–316Crossref, Medline, Google Scholar
21 Ironson G, Freund B, Strauss JL, et al: Comparison of two treatments for traumatic stress: a community-based study of EMDR and prolonged exposure. J Clin Psychol 2002; 58:113–128Crossref, Medline, Google Scholar
22 Hyer L, Brandsma JM: EMDR minus eye movements equals good psychotherapy. J Trauma Stress 1997; 10:515–522Medline, Google Scholar
23 Foa EB, Riggs DS, Dancu CV, et al: Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress 2002; 6:459–473Crossref, Google Scholar
24 Shapiro F: Eye Movement Desensitization and Reprocessing: Basic Principles, Protocols, and Procedures, 2nd ed. New York, Guilford, 2001Google Scholar
25 University College London, Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spmGoogle Scholar
26 Talairach J, Tournoux P: Co-Planar Stereotactic Atlas of the Human Brain. Stuttgart, Beorg Thieme Verlag, 1988Google Scholar
27 Brett M: Smoothing tutorial at http://www.mrc-cbu.cam.ac.uk/Imaging/smoothing.html.Google Scholar
28 Gündel H, O’Connor MF, Littrell L, et al: Functional neuroanatomy of grief: an fMRI study. Am J Psychiatry 2003; 160:1946–1953Crossref, Medline, Google Scholar
29 Servos P, Osu R, Santi A, et al: The neural substrates of biological motion perception: an fMRI study. Cereb Cortex 2002; 12:772–782Crossref, Medline, Google Scholar
30 Malouin F, Richards CL, Jackson PL, et al: Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp 2003; 19:47–62Crossref, Medline, Google Scholar
31 Moll J, de Oliveira-Souza R, Bramati IE, et al: Functional networks in emotional moral and nonmoral social judgments. Neuroimage 2002; 16:696–703Crossref, Medline, Google Scholar
32 Shipp S: The functional logic of cortico-pulvinar connections. Philos Trans R Soc Lond B Biol Sci 2003; 358:1605–1624Crossref, Medline, Google Scholar
33 Bremner JD, Innis RB, Southwick SM, et al: Decreased benzodiazepine receptor binding in prefrontal cortex in combat-related posttraumatic stress disorder. Am J Psychiatry 2000; 157:1120–1126Crossref, Medline, Google Scholar
34 Bremner JD: Neuroimaging studies in post-traumatic stress disorder. Curr Psychiatry Rep 2002; 4:254–263Crossref, Medline, Google Scholar
35 Xing GQ, Russell S, Webster MJ, et al: Decreased expression of mineralocorticoid receptor mRNA in the prefrontal cortex in schizophrenia and bipolar disorder. Int J Neuropsychopharmacol 2004:1-11Google Scholar
36 Brody AL, Barsom MW, Bota RG, et al: Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Semin Clin Neuropsychiatry 2001; 6:102–112Crossref, Medline, Google Scholar
37 Bremner JD, Vythilingam M, Ng CK, et al: Regional brain metabolic correlates of alpha-methylparatyrosine-induced depressive symptoms: implications for the neural circuitry of depression. JAMA 2003; 289:3125–3134Crossref, Medline, Google Scholar
38 Brody AL, Saxena S, Mandelkern MA, et al: Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol Psychiatry 2001; 50:171–178Crossref, Medline, Google Scholar
39 Goel V, Dolan RJ: Explaining modulation of reasoning by belief. Cognition 2003; 87:B11-22Google Scholar
40 Goel V, Dolan RJ: Reciprocal neural response within lateral and ventral medial prefrontal cortex during hot and cold reasoning. Neuroimage 2003; 20:2314–2321Crossref, Medline, Google Scholar
41 Debener S, Beauducel A, Nessler D, et al: Is resting anterior EEG alpha asymmetry a trait marker for depression? Neuropsychobiology 2000; 41:31–37Crossref, Medline, Google Scholar
42 Bench CJ, Friston KJ, Brown RG, et al: The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychol Med 1992; 22:607–615Crossref, Medline, Google Scholar
43 Bruder GE, Quitkin FM, Stewart JW, et al: Cerebral laterality and depression: differences in perceptual asymmetry among diagnostic subtypes. J Abnorm Psychol 1989; 98:177–186Crossref, Medline, Google Scholar
44 Davidson RJ: Anterior cerebral asymmetry and the nature of emotion. Brain Cogn 1992; 20:125–151Crossref, Medline, Google Scholar
45 Davidson RJ: Parsing affective space: perspectives from neuropsychology and psychophysiology. Neuropsychology 1993; 7:464–475Crossref, Google Scholar
46 Flor-Henry P: Cerebral Basis of Psychopathology. Boston, John Wright, 1983Google Scholar
47 Heller W: Gender differences in depression: perspectives from neuropsychology. J Affect Disord 1993; 29:129–143Crossref, Medline, Google Scholar
48 Schwartz GE, Davidson RJ, Maer F: Right hemisphere lateralization for emotion in the human brain: interactions with cognition. Science 1975; 19:286–288Crossref, Google Scholar



