Impaired Perception of Affective Prosody in Remitted Patients With Bipolar Disorder
Psychiatric disorders associated with poor social functioning, like schizophrenia, have also been associated with deficits in the recognition of facial expressions, 5 – 14 as well as of affective prosody. 7 , 10 , 11 , 15 – 17 In fact, such deficits appear to contribute directly to social dysfunction in schizophrenia by leading to impaired communication and difficulties in interpersonal relations, occupational functioning, and grooming/hygiene. 11 , 18 – 20 Despite the findings suggesting that patients with bipolar disorder present a high incidence of psychosocial difficulties even during remission, 21 – 24 which contribute significantly to poor prognosis, few studies have examined facial affect perception in patients with bipolar disorder, and even fewer studies have addressed affective prosody recognition in bipolar disorder.
Findings regarding facial affect recognition in patients with bipolar disorder have been inconsistent. Several studies of patients with bipolar disorder, currently manic or mixed, reported difficulties in decoding facial emotions. 25 – 28 Those studies examining facial affect recognition in euthymic patients, however, have reported inconsistent findings. Currently euthymic 5 or clinically stable, 29 although not remitted, patients with bipolar disorder, were found to be impaired on facial affect recognition tests. In contrast, a mixed group of patients with affective psychoses, 10 suffering from both depressive and bipolar disorder, or bipolar disorder in remission, 26 , 30 were not impaired in facial affect processing. Finally, in another study, a group of euthymic patients with bipolar disorder showed enhanced recognition of facial expressions of disgust compared with a group of healthy comparison subjects. 31
Very few studies have explored the perception of prosody, either affective or semantic, in bipolar disorder. Patients with mania performed more poorly than healthy participants on affective, but not stress (entailing decisions about semantic meaning), prosody comprehension. 32 Remitted patients with general affective psychoses, however, performed as well as healthy individuals on linguistic and affective prosody, and, consequently, there were no differences in specific emotion categories between the two groups. 10
Our purpose in undertaking the present study was to investigate the ability of remitted patients with bipolar disorder I to perceive affective prosody and to explore the specific emotions that might be troublesome for them. Despite the inconsistent findings reported previously, we expected that patients with bipolar disorder I, even in remission, would demonstrate difficulties in affective prosody perception, given their continued difficulties in social functioning. We had no basis on which to predict which particular emotions, however, would pose the greatest difficulty for these patients.
METHOD
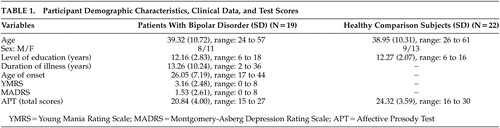
Participants comprised 19 (eight men) remitted patients with a diagnosis of bipolar disorder I and 22 (nine men) healthy subjects. Participants in the two groups gave their informed consent and experimenters adhered to international ethical standards relating to research participation. Patients with bipolar disorder were recruited from the outpatient services of two university psychiatric departments, while the healthy participants were recruited from the community; frequency matching was used in order to match patients and healthy subjects (case-controlled study). Patients had a mean age of 39.32 (SD=10.72, range=24 to 57) years and a mean education level of 12.16 (SD=2.83, range=6 to 18) years, while healthy individuals had a mean age of 38.95 (SD=10.72, range=26 to 61) years and a mean education level of 12.27 (SD=2.07, range=6 to 16) years. The mean age of onset of bipolar disorder was 26.05 (SD=7.19, range=17 to 44) years and their mean duration of illness was 13.26 (SD=10.24, range=2 to 36) years.
All patients were diagnosed according to DSM-IV criteria. 33 Diagnosis was confirmed with the Greek version (translation-adaptation to the Greek language by S. Beratis) of the Mini International Neuropsychiatric Interview (MINI). 34 In order to ensure symptom recovery, patients were included only if they had a score of eight or less on the Montgomery-Asberg Depression Rating Scale (MADRS) 35 and the Young Mania Rating Scale (YMRS). 36 Their mean score on the MADRS was 1.53 (SD=2.61, range=0 to 8) and on the YMRS was 3.16 (SD=2.48, range=0 to 8). Diagnoses and ratings of psychopathology were conducted by a psychiatrist (V.P.B.).
Exclusion criteria for both groups included neurological and developmental disorders, a history of head injury, alcohol or drug abuse during the 6-month period prior to testing, and any physical illness that may have affected the participants’ cognitive performance. Additional criteria for healthy participants were a history of a psychiatric disorder or treatment and a family history of psychosis. All healthy participants were screened with a semistructured interview by one of the experimenters (T.T.) before entering the study under the close supervision of the first and second authors (V.P.B., M.H.K.).
All of the patients were receiving the appropriate medication, antiepileptics, lithium, atypical antipsychotics, and antidepressants, either as monotherapy or in various combinations, to ensure mood stabilization at the time of the study: 14 were on an atypical antipsychotic, two on a classical antipsychotic, 14 on a mood stabilizer, and three on an antidepressant.
Demographic characteristics of the two groups, as well as patient clinical data, are presented in Table 1 .
 |
Affective Prosody Test (APT)
In this test, 30 audiorecorded sentences of emotionally neutral content (e.g., “Today is Wednesday”) were presented with prosodic intonation by a male actor portraying one of the basic emotions (happiness, sadness, surprise, fear, and anger, as well as neutral intonation) with five examples of each emotion. 37 The participants had a list of the options in front of them and made their choice after hearing each sentence. A training trial, in which participants heard the same sentence repeated with each of the above six prosodic intonations, preceded the experiment.
The psychometric properties of the APT have been demonstrated previously. 37 It has high internal consistency for the Greek population; its validity was also established, as it differentiated healthy adults from stroke patients. Finally, performance on the APT was influenced by education and age (decreased performance was associated with lower levels of education and greater age) but not by gender.
Statistical Analyses
Group comparisons (patients with bipolar disorder and healthy comparison subjects) were conducted with two-tailed t tests, for continuous demographic data, and with chi-square tests, for categorical data. Repeated measures analyses of variance (ANOVAs) were calculated, with group (patients with bipolar disorder, comparison subjects) and gender (male, female) as the between-group factors, and the six emotion categories (happiness, sadness, surprise, fear, anger, and neutral) as the within-subject factors. As the distribution of the six emotions were generally not normal, planned pairwise comparisons between the two gender groups were conducted with Mann-Whitney tests. The relationship between performance on the six emotion categories with clinical variables (residual symptoms of mania and depression, age of onset, and duration of illness) was explored with two-tailed Spearman correlation coefficients.
RESULTS
Patients with bipolar disorder and healthy individuals did not differ significantly in age [ t (39) = 0.11, p=0.91], level of education [t (39) = −0.08, p=0.94], and male to female ratio [chi-square(1) = 0.06, p=0.94] ( Table 1 ).
Repeated ANOVAs revealed a significant main effect for group ( F [1, 37] = 6.72, p=0.014), with patients performing more poorly than comparison subjects. The mean accuracy of the patients on the APT was 69.47% (SD=13.35) and that of healthy comparison subjects was 81.06% (SD= 11.97). Furthermore, there was a significant main effect for emotion categories ( F [5, 185] = 7.39, p≤0.001) but no interaction of group × emotion categories ( F [5, 185] = 0.23, p=0.95). In fact, the rank order of the six emotions from highest to lowest mean scores was similar in both groups. For the healthy participants the order was: neutral, happiness, fear, surprise, and anger/sadness, while for the bipolar patients the order was: neutral, happiness, surprise, anger, fear, and sadness.
The three-way interaction of group × gender × emotion categories was significant ( F [5, 185] = 3.95, p=0.002), suggesting that female and male patients presented a different deficit profile with respect to the six prosody emotions. Women with bipolar disorder performed more poorly than healthy women on fear (U=24.00, p=0.005) and surprise (U=37.00, p=0.047) but not on happiness (U=40.50, p=0.072), sadness (U=47.00, p=0.17), anger (U=50.00, p=0.23), and neutral intonation (U=39.00, p=0.063). On the other hand, men with bipolar disorder did not differ in their performance from healthy men in any of the six emotion categories (fear: U=28.00, p=0.48; happiness: U=31.00, p=0.67; surprise: U=33.00, p=0.82; sadness: U=27.00, p=0.42; anger: U=21.00, p=0.17; neutral intonation: U=35.50, p=0.96).
There was only a marginal positive correlation between residual depression symptoms, as measured by the MADRS, and performance on fear ( r [19]=0.43, r =0.066) and neutral intonation ( r [19]=−0.44, r =0.058).
DISCUSSION
Remitted patients with bipolar disorder I in the present study were impaired in the perception of affective prosody, as compared with age-, gender-, and education-matched healthy individuals. We also found an unexpected gender difference in the patient group, wherein female, but not male patients were impaired on affective prosody perception. With regard to each emotion type separately, female patients performed poorly on fear and surprise. The different deficit pattern that we found between male and female patients could not be attributed to differences in demographic or clinical characteristics between the sexes.
Our results contradict the findings of at least one investigation of affective prosody in patients with schizophrenia and patients with affective psychoses. 10 In this study, remitted patients with affective psychoses performed as well as healthy individuals on affective prosody. The mixed affective psychoses group, however, consisted of 23 remitted patients, 10 patients with major depression and 13 patients with bipolar disorder. In the same study, the affective psychoses group was not impaired on any specific emotions relative to healthy comparison subjects.
In contrast, our findings of impaired affective prosody perception (particularly fear perception) in remitted patients with bipolar disorder are in accordance with other reports. More specifically, our results are consistent with those indicating poor overall affective processing 5 in remitted patients with bipolar disorder. Also, at least one study has indicated specific difficulties in the perception of fear (when presented in the visual modality) 29 in a group of remitted patients with bipolar disorder.
The present findings are consistent with the notion that many of the symptoms experienced by patients with bipolar disorder, including irritability, distractibility, and emotional lability, would appear to be associated with abnormalities in emotion processing, including the experience of emotions of inappropriately high intensity in relation to the context in which they occur, and an inability to regulate mood. 38 Our findings of impaired affect perception, regardless of stimulus modality, during the remission phase of bipolar disorder suggest that this difficulty is not restricted to manic or mixed episodes, but represents a more enduring deficit that might predispose patients to subsequent relapses. In fact, even during remission, patients with bipolar disorder have a high incidence of psychosocial difficulties. These difficulties may contribute significantly to poor prognosis, 21 – 24 and may even be as severe as those of patients with schizophrenia. 23 It has been estimated that 30% to 50% of largely remitted patients fail to attain premorbid levels of psychosocial functioning. 39 In patients with schizophrenia, affective prosody impairment was found to be related to dysfunction in occupational performance. 11 Similarly, inaccurate “affect recognition,” a composite index based on measures of vocal and facial affect identification, was found to be associated with poor interpersonal relationships. 20 It is possible that impaired affective prosody recognition in remitted patients with bipolar disorder might contribute to the interpersonal and occupational problems observed in those patients even during interepisode recovery, especially for female patients.
In summary, patients with bipolar disorder, currently in remission, presented significant impairment in the recognition of affective prosody. This impairment was specific to female patients and certain emotions, such as fear and surprise. Future studies should explore the consequences of these deficits on the social and interpersonal functioning of remitted patients with bipolar disorder, especially female patients, more directly.
1 . Green MF, Kern RS, Robertson MJ, et al: Relevance of neurocognitive deficits for functional outcome in schizophrenia, in Cognition in Schizophrenia: Impairments, Importance and Treatment Strategies. Edited by Sharma T, Harvey P. New York, Oxford University Press, 2000, pp 178–192Google Scholar
2 . Adolphs R: Neural systems for recognizing emotion. Curr Opin Neurobiol 2002; 12:169–177Google Scholar
3 . Adolphs R, Damasio H, Tranel D: Neural systems for recognition of emotional prosody: a 3D lesion study. Emotion 2002; 2:23–51Google Scholar
4 . Edwards J, Jackson HJ, Pattison PE: Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev 2002; 22:789–832Google Scholar
5 . Addington J, Addington D: Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr Res 1998; 32:171–181Google Scholar
6 . Mandal MK, Pandey R, Prasad AB: Facial expressions of emotions and schizophrenia: a review. Schizophr Bull 1998; 24:399–412Google Scholar
7 . Shaw RJ, Dong M, Lim KO, et al: The relationship between affect expression and affect recognition in schizophrenia. Schizophr Res 1999; 37:245–250Google Scholar
8 . Habel U, Gur RC, Mandal MK, et al: Emotional processing in schizophrenia across cultures: standardized measures of discrimination and experience. Schizophrenia Res 2000; 42:57–66Google Scholar
9 . Kohler CG, Bilker W, Hagendoorn M, et al: Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biological Psychiatry 2000; 48:127–136Google Scholar
10 . Edwards J, Pattison PE, Jackson HJ, et al: Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr Res 2001; 48:235–253Google Scholar
11 . Hooker C, Park S: Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res 2002; 112:41–50Google Scholar
12 . Kohler CG, Turner TH, Bilker WB, et al: Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry 2003; 160:1768–1774Google Scholar
13 . Kosmidis MH, Bozikas VP, Giannakou M, et al: Impaired affect recognition without visuoperceptual deficits in schizophrenia. J Int Neuropsychol Soc 2003; 9:512Google Scholar
14 . Kosmidis MH, Bozikas VP, Giannakou M, et al: Impaired emotion perception in schizophrenia: a differential deficit. Psychiatry Res 2007; 149:279–284Google Scholar
15 . Leentjens AFG, Wielaert SM, van Harskamp F, et al: Disturbances of affective prosody in patients with schizophrenia; a cross sectional study. J Neurol Neurosurg Psychiatry 1998; 64:375–378Google Scholar
16 . Ross ED, Orbelo DM, Cartwright J, et al: Affective-prosodic deficits in schizophrenia: profiles of patients with brain damage and comparison with relation to schizophrenic symptoms. J Neurol Neurosurg Psychiatry 2001; 70:597–604Google Scholar
17 . Bozikas VP, Kosmidis MH, Anezoulaki D, et al: Impaired perception of affective prosody in schizophrenia. J Neuropsychiatry Clin Neurosci 2006; 18:81–85Google Scholar
18 . Mueser KT, Doonan R, Penn DL, et al: Emotion recognition and social competence in chronic schizophrenia. J Abnorm Psychol 1996; 105:271–275Google Scholar
19 . Penn DL, Spaulding W, Reed D, et al: The relationship of social cognition to ward behaviour in chronic schizophrenia. Schizophr Res 1996; 20:327–335Google Scholar
20 . Poole JH, Tobias FC, Vinogradov S: The functional relevance of affect recognition errors in schizophrenia. J Int Neuropsychol Soc 2000; 6:649–658Google Scholar
21 . Coryell W, Scheftner W, Keller M, et al: The enduring psychosocial consequences of mania and depression. Am J Psychiatry 1993; 150:720–727Google Scholar
22 . Cooke RG, Robb JC, Young LT, et al: Well-being and functioning in patients with bipolar disorder assessed using the MOS 20-item short form (SF-20). J Affect Disord 1996; 39:93–97Google Scholar
23 . Dickerson FB, Sommerville J, Origoni AE, et al: Outpatients with schizophrenia and bipolar disorder I: do they differ in their cognitive and social functioning? Psychiatry Res 2001; 102:21–27Google Scholar
24 . Abbod Z, Sharkey A, Webb M, et al: Are patients with bipolar affective disorder socially disadvantaged? a comparison with a control group. Bipolar Disord 2002; 2:243–248Google Scholar
25 . Getz GE, Shear PK, Strakowski SM: Facial affect recognition deficits in bipolar disorder. J Int Neuropsychol Soc 2003; 9:623–632Google Scholar
26 . Lembke A, Ketter TE: Impaired recognition of facial emotion in mania. Am J Psychiatry 2002; 159:302–304Google Scholar
27 . McClure EB, Pope K, Hoberman AJ, et al: Facial expression recognition in adolescents with mood and anxiety disorders. Am J Psychiatry 2003; 160:1172–1174Google Scholar
28 . Lennox BR, Jacob R, Calder AJ, et al: Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychol Med 2004; 34:795–802Google Scholar
29 . Yurgelun-Todd DA, Gruber SA, Kanayama G, et al: fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord 2000; 2:237–248Google Scholar
30 . Venn HR, Gray JM, Montagne B, et al: Perception of facial expressions of emotion in bipolar disorder. Bipolar Disord 2004; 6:286–293Google Scholar
31 . Harmer CJ, Grayson L, Goodwin GM: Enhanced recognition of disgust in bipolar illness. Biol Psychiatry 2002; 51:298–304Google Scholar
32 . Murphy D, Cutting J: Prosodic comprehension and expression in schizophrenia. J Neurol Neurosurg Psychiatry 1990; 53:727–730Google Scholar
33 . American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Press, 1994Google Scholar
34 . Sheehan DV, Lecrubier Y, Sheehan KH, et al: The mini-international neuropsychiatric (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl20):22–33Google Scholar
35 . Montgomery SA, Asberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–289Google Scholar
36 . Young RC, Biggs JT, Ziegler VE, et al: A rating scale for mania: relaibility, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Google Scholar
37 . Hiou K, Vagia A, Haritidou E, et al: Affect perception as a cognitive function: validity and clinical application of a neuropsychological test battery in healthy individuals and patients with brain lesions. Psychol (in Greek) 2004; 11:388–401Google Scholar
38 . Phillips ML, Drevets WC, Rauch SL, et al: Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry 2003; 54:515–528Google Scholar
39 . Goodwin FK, Jamison KR: Manic-depressive illness. New York, Oxford University Press, 1990Google Scholar



