HIV-Associated Episodic Memory Impairment: Evidence of a Possible Differential Deficit in Source Memory for Complex Visual Stimuli
Concordant with the prevalence of HIV-associated neuropathologies, neurocognitive impairment is also common in HIV infection, 9 particularly in milder forms, which affect up to 50% of this population. 10 The preferential impact of HIV infection on the frontostriatal circuits results in a prototypical pattern of deficits, including slowed information processing efficiency, executive dysfunction, and deficient episodic memory encoding and retrieval. 11 Regarding the episodic memory deficits more specifically, a characteristic pattern of diminished free recall contrasted with relatively intact retention and recognition (i.e., limited evidence of “rapid forgetting”) is evident in HIV. 12 These encoding and retrieval deficits have been linked to limited use of higher-level organizational strategies, such as semantic clustering. 12 Given that retention is commonly preserved, the diminished use of such strategies indicates that the memory profile in HIV infection is consistent with dysfunction of the strategic (i.e., executive) aspects of encoding and retrieval. Support for this hypothesis is provided by numerous prior studies of episodic memory in HIV. 13 – 16
A specific aspect of episodic memory that is dependent on frontal systems, but has yet to be evaluated in HIV, is source memory. 17 , 18 Episodic memory refers to memory for experienced events, 19 which includes not only information about the content of the event, known as item memory , but also the context in which the event occurred, or source memory . 20 In fact, Craik et al. 21 suggested that a hallmark of episodic memory is that the individual recollects the circumstances around which the event in question was acquired. Importantly, source memory encompasses not only the information about the source from which the information was acquired, but also contextual information such as temporal sequence, spatial location, and perceptual aspects (e.g., presentation modality). 20 , 22 Item and source memory are singly dissociable, such that an individual may remember the content, or facts, of an event but may not recall the specific circumstances around which the information was acquired (e.g., the gender of the informant). For example, when one person relates a story to another person, the latter individual may recall the content of the story (e.g., intact item memory) but may not remember details about the storyteller (e.g., gender) or the specific time or location at which the story was told (e.g., before or after dinner).
It has been posited that source memory is more strongly associated with frontal systems than is item memory, which is more reliant on medial temporal structures. Evidence for the single dissociation of item and source memory has been demonstrated in several populations with frontal systems dysfunction, including healthy older adults, patients with frontal lobe lesions, and individuals with movement disorders. 23 – 26 For example, a recent investigation revealed impaired source, but not item memory for visual material, in individuals with Huntington’s disease as compared to healthy adults. 26 The notion that item and source memory rely on somewhat different neural substrates is also supported by several functional MRI (fMRI) studies that reveal altered BOLD activity in the prefrontal cortex region, but not medial temporal regions, during source memory tasks. 27 – 30
Given the preferential effects of HIV-related neuropathologies on frontal systems and the associated executive dysfunction and deficient strategic encoding and retrieval, it is plausible that source memory would be disproportionately impaired relative to item memory in this population. To our knowledge, however, source memory has not previously been examined in individuals with HIV infection. As such, the current study examined item and source memory performance in HIV-seropositive individuals and a seronegative comparison group. It was hypothesized that HIV seropositivity would be associated with source memory impairment, perhaps even to a greater extent than item memory. It was further hypothesized that source memory deficits would correlate with impairment on measures of executive functioning, working memory, and other higher-level episodic memory encoding indices (e.g., semantic clustering), but not with measures of semantic memory (e.g., confrontational naming).
METHODS
Participants
Sixty seropositive individuals and 35 seronegative comparison participants were included in the study. HIV status was determined by enzyme-linked immunosorbent assays and confirmed by a Western Blot test. Individuals with histories of color-blindness, neurological disease (e.g., seizure disorders, closed head injuries with loss of consciousness greater than 15 minutes, and CNS neoplasms or opportunistic infections), severe psychiatric illness (e.g., schizophrenia or bipolar disorder), or verbal IQ estimates less than 70 (based on the Wechsler Test of Adult Reading 31 ) were excluded. Individuals with diagnoses of substance-related disorders within 6 months of evaluation as determined by the Composite International Diagnostic Interview (CIDI version 2.132) were also excluded. To confirm recent abstinence, participants underwent a urine toxicology screen for illicit drugs (other than marijuana) on the day of evaluation. The rationale for inclusion of individuals with positive marijuana toxicology screens (HIV+, n=9; seronegative comparison, n=2) is that marijuana is detectable up to 30 days after last use and positive toxicology results can be produced by commonly prescribed medications for persons with HIV (e.g., efavirenz, marinol).
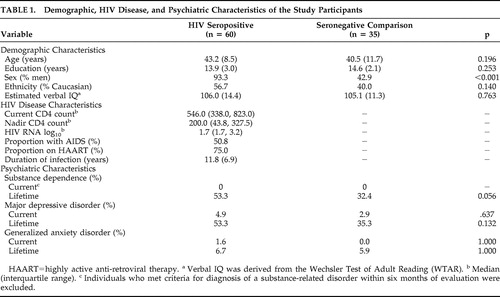
Table 1 summarizes the demographic, HIV disease, and psychiatric characteristics of the overall study sample. Given that HIV infection is commonly accompanied by numerous other CNS risk factors (e.g., substance dependence), we endeavored to recruit a seronegative comparison sample that had comparable exposure to such confounds in an effort to better isolate the specific effects of HIV on item and source memory. As such, the HIV and seronegative comparison groups were comparable on demographic (i.e., age, sex, education, and ethnicity) and psychiatric (i.e., lifetime diagnoses of substance dependence, current and lifetime diagnoses of major depressive disorder, and generalized anxiety disorder) variables known to influence neuropsychological performance whenever possible.
 |
Materials and Procedure
All participants provided written, informed consent, and completed comprehensive neuropsychological, psychiatric, and medical research assessments for which they received financial compensation.
Item and Source Memory
Participants were administered an experimental measure of working memory, the Self-Ordered Pointing Test, 33 which was modified to embed the visual item and source memory tasks. The modified version included two color trials in which visual stimuli (i.e., patterned designs) were presented with 1/8” colored borders (i.e., either red or black). The order of presentation of the color trials was randomized for each participant. For the purposes of this test, the designs represented the item information and the color of the border represented the source information. For each of the two trials, participants were presented paper stimulus pages with 12 designs on each page. Figure 1 , panel A displays a selection of sample designs similar to those that appeared on the stimulus pages for the black and red trials. There were 12 stimulus pages for each color trial, and each of the 12 designs appeared on each page, but was located in different, randomly selected positions in the array on each page. The 12 pages were presented sequentially, and the participants were instructed to point to a different design on each page. That is, as each page was presented, the participant was supposed to point to a design that he or she had not pointed to previously. After presentation of one color trial was finished, the other color trial was administered in the same format, with a different set of 12 designs. There was no time limit for pointing to a design or for completion of the test overall, and no feedback was given throughout test administration.
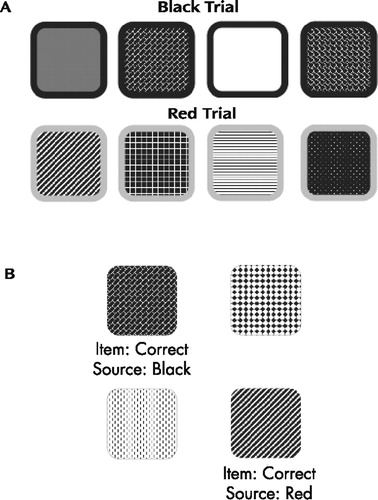
After presentation of the two color trials there was a 5-minute delay, during which time the participants were administered a brief sentence-reading test (described in detail below). Following the delay, a forced choice recognition trial was administered, in which each design was presented without its colored border and was paired with a foil (i.e., novel) design. Figure 1 , panel B displays two sample items similar to those shown to the participants during the forced choice recognition trial. The item memory task was to identify which design had been previously presented. The source memory task was to identify whether the colored border on that design had been red or black. The number of errors for each task was recorded, with the 12 possible errors for each task summed across the two trials for a total maximum error score of 24 for both the item and source memory tasks, respectively. A third score was coded such that source errors were only counted when the item response was correct (i.e., the maximum number of source errors was dependent upon the number of correct item responses).
All participants also received a modified version of the verbal Source and Item Memory Test. 34 During the presentation phase, the participant was asked to read 10 sentences (i.e., item information) aloud to the examiner, each of which was attributed to one of five identified individuals (i.e., source information: grandmother, father, mother, son, or daughter). For example, one of the sentences reads, “Grandmother says: I like baked beans on toast.” As with the visual source memory task, participants were not told in advance that they would be asked to recall either the item or source information. After a 5-minute delay (during which participants completed the visual source memory recognition trial described above), the participant was presented with a 10-item posttest paper and pencil quiz. Each test item consisted of three sentences (one previously presented stimulus sentence and two novel foil sentences) and three of the five individuals (e.g., grandmother, father, mother, son, and/or daughter). The item memory task was to identify which one of the three sentences had been presented earlier. The source memory task was to identify which of the three individuals listed was originally linked to that sentence. The number of item and source errors was recorded and summed, for a maximum score of 10, respectively. Again, a more stringent source memory error score was coded such that source errors were only recorded when the item response was correct.
General Neuropsychological Battery
In addition to the item and source memory tasks described above, participants were administered several other standardized neuropsychological tests, including Tower of London–Drexel; 35 Trail Making Test Parts A and B; 36 digit span subtest from the Wechsler Adult Intelligence Scale, 3rd edition (digits backward; WAIS-III 37 ); the California Verbal Learning Test, 2nd edition (semantic clustering and across-list intrusions 38 ); Rey-Osterreith Complex Figure Test (copy trial planning score 39 ); Boston Naming Test; 40 and the Famous Faces subtest from the Kaufman Adolescent and Adult Intelligence Test. 41
Medical and Psychiatric Assessments
All participants received a full research neuromedical assessment, which included a review of medications, medical history and current symptoms, a complete physical and neurological evaluation, and a blood draw. CD4+ lymphocytes were counted in blood using flow cytometry and HIV RNA levels were quantified in plasma using RT-PCR (Amplicor, Roche Diagnostics, Indianapolis). Participants also underwent a structured psychiatric assessment using the CIDI, which provided lifetime and current (i.e., within 1 month of evaluation) diagnoses of major depressive disorder, generalized anxiety disorder, and substance-related disorders as per DSM-IV criteria. 42
Data Analyses
The primary dependent variables of interest were all non-normally distributed as assessed with the Shapiro-Wilk W test (p<0.05 in all cases). Accordingly, all subsequent analyses were undertaken using a nonparametric approach, including Wilcoxon Rank Sums test for between-group comparisons and Spearman’s rho (ρ) for within-group correlational analyses. Unbiased Cohen’s d values were generated to estimate the effect sizes associated with the between-group analyses. By convention, Cohen’s d values of 0.2, 0.5, and 0.8 correspond to small, medium, and large effect sizes, respectively. To minimize the risk of type I error, correlational analyses with the standard cognitive measures were restricted to only those source memory variables on which the HIV group was impaired relative to the seronegative comparison sample. The critical alpha level was set at 0.05 for all analyses.
RESULTS
There were no significant effects of sex on any dependent variable in either the HIV+ or seronegative comparison groups (p>0.10). As shown in Figure 2 , trend-level differences were observed between the HIV+ and seronegative comparison groups on the verbal item (p=0.05, d=0.50) and source (p=0.06, d=0.42) memory variables. However, null findings emerged when the analysis was restricted to only those source memory errors that occurred in the context of correct item memory performance (p=0.51, d=0.12). On the visual Self Ordered Pointing Task, the HIV+ (median =4.0, interquartile range =2.0, 6.0) and seronegative comparison (median =4.0, interquartile range =3.0, 5.0) groups did not differ in the total number of errors (p=0.44, d=0.23). In contrast, the HIV+ group committed more source (p=0.02, d=0.49), but not item (p=0.83, d=0.10) memory errors on the visual Self Ordered Pointing Task relative to the seronegative comparison group. This significant result also survived the more restricted analysis of only those source memory errors that occurred after a correct item memory response (p=0.02, d=0.52). Although the non-normal distribution of our data did not allow us to formally examine a possible interaction between HIV serostatus and item/source memory, we conducted a follow-up between-group analysis using a difference score calculated by subtracting visual item errors from the corrected visual source memory errors. Results revealed a trend-level effect such that the HIV group (median =−7.5, interquartile range =−10.0, −3.0) had disproportionately larger difference score (p=0.05, d=0.28) relative to the seronegative comparison sample (median =−4.0, interquartile range =−7.0, −3.0).
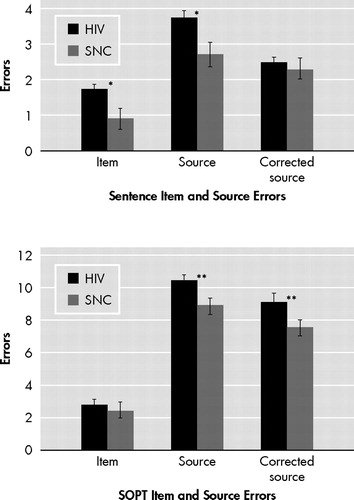
Data represent the mean with SEM error bars. SOPT=Self Ordered Pointing Task. *p<0.10; **p<0.05
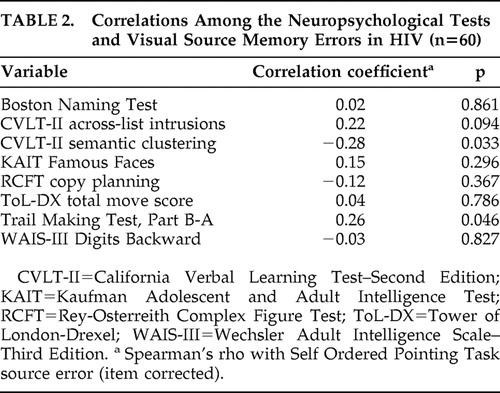
Table 2 shows that, within the HIV group, the corrected visual source memory errors were significantly associated with Trail Making Test B-A impairment and lower semantic clustering on the California Verbal Learning Test-II (p<0.05 in all cases), and corresponded at a trend-level with increased across-list intrusion errors on the California Verbal Learning Test (p<0.10). Within the seronegative comparison sample, the corrected visual source memory errors were significantly correlated with poorer performance on the Tower of London (i.e., total move score), WAIS-III Digits Backward (p<0.05 in all cases), and California Verbal Learning Test-II across-list intrusions ( Table 3 ), as well as with Trail Making Test B-A impairment at a trend-level (p=0.07). In neither group were the corrected visual source memory errors correlated with the Rey Complex Figure Test, Boston Naming Test, or Kaufman Adolescent and Adult Intelligence Test Famous Faces (p>0.10 in all cases).
 |
 |
No relationships were observed between the corrected visual source memory errors and HIV disease severity, including duration of infection, nadir or current CD4, HIV RNA in plasma or CSF, AIDS diagnoses, or antiretroviral therapy (p>0.10 in all cases). Importantly, there were no main effects of lifetime histories of major depressive, generalized anxiety or substance-related disorders on corrected visual source memory errors (p>0.10 in all cases), nor were there any significant interactions between these three conditions and HIV serostatus (p>0.10 in all cases). Moreover, there were no main effects or interaction effects for a positive marijuana toxicology screen, current major depressive disorder, or current generalized anxiety disorder (p>0.10 in all cases).
DISCUSSION
The aim of this study was to examine the nature, extent, and cognitive correlates of possible source memory deficits in persons living with HIV infection. Given the predominant frontostriatal neuropathological sequelae of HIV, it was expected that seropositivity would be associated with poorer source memory, even to a greater extent than item memory. Results provided partial support for this hypothesis, revealing impaired source, but not item memory, in an HIV seropositive group relative to seronegative comparison participants on a complex visual memory task. This finding, which was associated with a medium effect size, indicates that the HIV sample was able to distinguish previously presented visual designs (i.e., item information) from foils with similar accuracy as the seronegative comparison participants, but nevertheless experienced difficulty recalling whether the borders on the designs had been red or black (i.e., source information). Importantly, the HIV-associated source memory deficit persisted even when a more conservative analysis was performed, which included only those source errors that occurred when the item memory response was correct. The HIV effect was more stringently identified through the use of a demographically and psychiatrically comparable seronegative comparison group (as these factors are known to affect neuropsychological performance). In fact, follow-up analyses further revealed that the apparent HIV-associated source memory deficit was not better explained by (or moderated by) demographics, depression, or substance abuse. Moreover, although nonnormal distributions precluded a parametric examination of this apparent interaction, a follow-up nonparametric analysis of the raw difference between visual item and source errors revealed a trend-level effect (p=0.05) that was associated with a small-to-medium effect size. This finding bolsters the contention that HIV is associated with a disproportionately larger deficit on source versus item memory for complex visual stimuli.
The observed pattern of correlations between visual source memory and putative measures of executive functions, working memory, and executive control during list learning provides support for the purported role of strategic processes in HIV-associated source memory impairment. Within the seronegative comparison sample, for example, visual source memory errors were associated with poorer performance on the Tower of London, WAIS-III Digits Backward, California Verbal Learning Test-II across-list intrusions, and Trail Making Test B-A. Similarly, in the HIV-infected group, greater visual source memory errors correlated with Trail Making Test B-A impairment and lower semantic clustering scores on the California Verbal Learning Test-II. The latter result is consistent with a study by Wegesin and colleagues, 43 which revealed that nondemented older adults who utilized a semantic clustering strategy demonstrated better source memory performance. Thus, the current visual source memory findings also converge with the considerable prior evidence of executive dyscontrol of encoding and retrieval within episodic memory in persons living with HIV infection. 14 The divergent validity of this working hypothesis was supported by the lack of correspondence between source memory and measures of semantic memory (i.e., Boston Naming Test and Kaufman Adolescent and Adult Intelligence Test Famous Faces).
A slightly different profile of results emerged on the verbal item and source memory tasks, which were both associated with trend-level effects suggestive of mild deficits in the HIV group. In other words, individuals in the HIV sample were generally less accurate in discriminating the sentences they had previously read aloud (i.e., item information), as well as in identifying the source (e.g., “grandmother”) to which the sentence had been attributed. Unlike visual source memory, however, the between-group differences disappeared when the analysis was restricted to only those verbal source errors that occurred after a correct item response, indicating that the verbal source memory difference was confounded by a deficit in item memory.
Several possible explanatory factors regarding the discrepant visual and verbal source memory findings must be considered. One possibility is that this discrepancy reflects lateralized HIV-associated brain dysfunction. This interpretation is unlikely, however, because as noted above, the neuropathophysiological effects of chronic HIV infection are not restricted to specific brain regions and do not generally gravitate to one hemisphere over another in the absence of CNS opportunistic infections, 2 which were a basis for exclusion in this study. Moreover, the extensive neuropsychological literature provides no evidence of lateralizing cognitive or motor sequelae of HIV-associated neuropathology. 11 Another possible explanation for the observed discrepancy between visual and verbal source memory is differential task difficulty. 44Figure 2 shows that all participants committed a larger proportion of errors on the visual source memory task as compared to the verbal test, which may indicate that the former is simply more sensitive than the latter due to its relatively greater level of difficulty.
Perhaps a more compelling explanation for the discrepant visual and verbal source memory results involves the cognitive mechanisms underlying task complexity (i.e., the cognitive demands of the foreground task) and centrality (i.e., the extent to which the source information is central to the foreground task). Regarding the issue of task complexity, the visual source memory test had a much more cognitively demanding foreground task (i.e., Self Ordered Pointing Task, which is a test of working memory) than did its verbal counterpart (i.e., simple oral reading). In fact, a post-hoc analysis revealed that general Self Ordered Pointing Task performance was strongly correlated with Digits Backwards in both the HIV+ (ρ=−0.59, p<0.0001) and seronegative comparison (ρ=−0.51, p=0.002) samples. Therefore, it is possible that the competing demands of the working memory task adversely impacted source encoding and retrieval for the visual test (i.e., border color), as has been demonstrated in healthy adults. 45 In fact, it has been previously shown that dual-task conditions disproportionately exacerbate HIV-associated neurocognitive impairment. 46 Turning next to the issue of task centrality, the source information for the visual task (i.e., border color) was much more peripheral than for the verbal task (i.e., the person to whom the sentence was attributed). Although participants were not explicitly instructed to remember which individual (e.g., “grandmother”) was paired with each sentence, the source information was nevertheless much more focal to the verbal foreground task (i.e., it was explicitly part of the sentence to be read). In contrast, the color of the design borders was much more peripheral, which ostensibly placed greater demands on strategic encoding and retrieval strategies. 47 Future studies in which task complexity and centrality are manipulated within a rigorous experimental design are needed to more fully understand the relative contributions of these factors to HIV-associated source memory deficits. The extent to which the observed deficits generalize to other aspects of contextual information (e.g., temporal sequence and other sensory modalities) also remains to be determined.
A few limitations of the current study should be considered in interpreting these data. Although the demonstration of source memory impairment is consistent with HIV-related frontal systems dysfunction, the veracity of this supposition is restrained by the inferential nature of the data. We cannot confidently rule out the contribution of medial temporal pathology to these findings, 48 , 49 which will require the incorporation of neuroimaging and/or neuropathological methods. Additionally, given that the sample was predominantly male, Caucasian, and evidenced generally well-controlled HIV disease, the results of this study may not generalize to other HIV populations (e.g., women, ethnic minorities, and persons with advanced disease). Furthermore, the lack of association between source memory and HIV disease characteristics in this study may simply reflect a restricted range of values. Finally, a common criticism of source memory tasks is that they are implicit by their very nature, which raises the problem of differential task complexity as compared to item memory (as was noted above regarding the verbal-visual discrepancy). As a result, one might argue that any differences observed are attributable to the fact that individuals are likely to make more errors on a difficult task than on an easy task. 44 This critique is particularly relevant when the item and source memory tasks are not psychometrically matched; that is, when item memory is examined with a forced-choice recognition format and source memory is evaluated with a free recall task that may have a greater number of response possibilities than the item task. Although the present study methodology partially combats that argument by using a recognition format with the same number of options for both tasks, the nature of the recognition trials (i.e., old/new for item memory and old/old for source memory) is nevertheless, by necessity, still discrepant.
Though not yet explicitly examined, there may be clinical implications of source memory impairment in persons with HIV infection. Deficient source memory would be most likely to manifest as mild problems in instrumental activities of daily living for individuals at a high level of functioning. For example, source memory failures could be problematic for individuals who are employed in high-level jobs or enrolled in an academic program, in which settings the source of learned information may be particularly relevant. Also, medical compliance is highly important for seropositive individuals (e.g., adherence to complex medication regimens), and failure to recall the source of pertinent medical information may be problematic. Though subtle, such effects may be increasingly common as more seropositive individuals remain medically stable due to effective treatment with highly active antiretroviral therapies (HAART), but nevertheless evidence mild neurocognitive impairment that may interfere with optimal daily living. 50
In sum, the current study provides the first evidence of impaired source memory for complex visual material in persons living with HIV infection. Given the predominantly frontostriatal neuropathology of HIV infection, this finding extends previous reports of source memory impairment in other populations with frontal systems dysfunction (e.g., aging). The present results also serve to characterize the nature of HIV-associated episodic memory impairment. In particular, the demonstration of a differential source memory deficit supplements the growing literature indicating that strategic aspects of encoding and retrieval are deficient in HIV infection. In light of the current findings, direct examination of the neural mechanisms of source memory in HIV (e.g., neuroimaging) and the potential implications of source memory impairment on everyday functioning warrant further investigation.
1. Davis LE, Hjelle BL, Miller VE, et al: Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 1992; 42:1736–1739Google Scholar
2. Gonzalez-Scarano F, Martin-Garcia J: The neuropathogenesis of AIDS. Nat Rev Immunol 2005; 5:69–81Google Scholar
3. Budka H. The neuropathology of HIV-associated brain disease, in The Neurology of AIDS, 2nd ed. Edited by Gendelman HE, Grant I, Everall I, et al. New York, Oxford University Press, 2005, pp 375–391Google Scholar
4. Ellis R, Langford D, Masliah E: HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 2007; 8:33–44Google Scholar
5. Wiley CA, Soontornniyomkij V, Radhakrishnan L, et al: Distribution of brain HIV load in AIDS. Brain Pathol 1998; 8:277–284Google Scholar
6. Stout JC, Ellis RJ, Jernigan TL, et al: Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Res Center Group. Arch Neurol 1998; 55:161–168Google Scholar
7. Chang L, Ernst T, Leonido-Yee M, et al: Perfusion MRI detects RCBF abnormalities in early stages of HIV-cognitive motor complex. Neurology 2000; 54:389–396Google Scholar
8. Chang L, Ernst T, Speck O, et al: Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry 2005; 162:361–369Google Scholar
9. Grant I, Atkinson JH, Hesselink JR, et al: Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections. Studies with neuropsychologic testing and magnetic resonance imaging Ann Intern Med 1987; 107:828–836Google Scholar
10. Heaton RK, Grant I, Butters N, et al: The HNRC 500: neuropsychology of HIV infection at different disease stages. J Int Neuropsychol Soc 1995; 1:231–251Google Scholar
11. Reger M, Welsh R, Razani J, et al: A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc 2002; 8:410–424Google Scholar
12. Murji S, Rourke SB, Donders J, et al: Theoretically derived CVLT subtypes in HIV-1 infection: internal and external validation. J Int Neuropsychol Soc 2003; 9:1–16Google Scholar
13. Carey CL, Woods SP, Rippeth JD, et al: Prospective memory in HIV-1 infection. J Clin Exp Neuropsychol 2006; 28:536–548Google Scholar
14. Delis DC, Peavy G, Heaton R, et al: Do patients with HIV-associated minor cognitive/motor disorder exhibit a “subcortial” memory profile? Evidence using the California Verbal Learning Test. Assessment 1995; 2:151–165Google Scholar
15. Gongvatana A, Woods SP, Taylor MJ, et al: Semantic clustering inefficiency in HIV-associated dementia. J Neuropsychiatry Clin Neurosci 2007; 19:36–42Google Scholar
16. Peavy G, Jacobs D, Salmon DP, et al: Verbal memory performance of patients with human immunodeficiency virus infection: evidence of subcortical dysfunction. J Clin Exp Neuropsychol 1994; 16:508–523Google Scholar
17. Evans FJ, Thorn WA: Two types of posthypnotic amnesia: recall amnesia and source amnesia. Int J Clin Exp Hypn 1966; 14:162–179Google Scholar
18. Schacter DL, Harbluk JL, McLachlan DR: Retrieval without recollection: an experimental analysis of source amnesia. J Verbal Learning and Verbal Behavior 1984; 23:593–611Google Scholar
19. Tulving E: Episodic and semantic memory, in Organization of Memory. Edited by Tulving E, Donaldson W. Oxford, England, Academic Press, 1972Google Scholar
20. Johnson MK, Hashtroudi S, Lindsay DS: Source monitoring. Psychol Bull 1993; 114:3–28Google Scholar
21. Craik FI, Morris LW, Morris RG, et al: Relations between source amnesia and frontal lobe functioning in older adults. Psychol Aging 1990; 5:148–151Google Scholar
22. Siedlecki KL, Salthouse TA, Berish DE: Is there anything special about the aging of source memory? Psychol Aging 2005; 20:19–32Google Scholar
23. Glisky EL, Polster MR, Routhieaux BC: Double dissociation between item and source memory. Neuropsychology 1995; 9:229–235Google Scholar
24. Hsieh S, Lee CY: Source memory in Parkinson’s disease. Percept Mot Skills 1999; 89:355–367Google Scholar
25. Janowsky JS, Shimamura AP, Squire LR: Source memory impairment in patients with frontal lobe lesions. Neuropsychologia 1989; 27:1043–1056Google Scholar
26. Pirogovsky E, Gilbert PE, Jacobson M, et al: Impairments in source memory for olfactory and visual stimuli in preclinical and clinical stages of Huntington’s disease. J Clin Exp Neuropsychol 2007; 29:395–404Google Scholar
27. Cansino S, Maquet P, Dolan RJ, et al: Brain activity underlying encoding and retrieval of source memory. Cereb Cortex 2002; 12:1048–1056Google Scholar
28. Dobbins IG, Foley H, Schacter DL, et al: Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron 2002; 35:989–996Google Scholar
29. Lundstrom BN, Ingvar M, Petersson KM: The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage 2005; 27:824–834Google Scholar
30. Slotnick SD, Moo LR, Segal JB, et al: Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res 2003; 17:75–82Google Scholar
31. Wechsler D: Wechsler Test of Adult Reading. San Antonio, Tex, Psychological Corporation, 2001Google Scholar
32. World Health Organization: Composite International Diagnostic Interview (CIDI), version 2.1. Geneva, WHO, 1998Google Scholar
33. Shimamura AP, Jurica PJ: Memory interference effects and aging: findings from a test of frontal lobe function. Neuropsychology 1994; 8:408–412Google Scholar
34. van Niekerk JK, Nielsen TA, Pasu S, et al: The development of new tests of source memory and a new approach to the testing of equivalence of parallel versions. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2004; 11:416–427Google Scholar
35. Culbertson WC, Zillmer EA: The Tower of London DX (TOLDX) Manual. North Tonawanda, NY, Multi-Health Systems, 2001Google Scholar
36. Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery, Theory and Clinical interpretation. Tucson, Ariz, Neuropsychology Press, 1985Google Scholar
37. Psychological Corporation: WAIS-III and WMS-III Technical Manual. San Antonio, Tex, The Psychological Corporation, 1997Google Scholar
38. Delis DC, Kramer JH, Kaplan E, et al: The California Verbal Learning Test, 2nd ed. San Antonio, Tex, The Psychological Corporation, 2000Google Scholar
39. Stern RA, Javorsky DJ, Singer EA, et al: The Boston Qualitative Scoring System for the Rey-Osterreith Complex Figure (BQSS): Manual. Lutz, Fla, Psychological Assessment Resources, 1999Google Scholar
40. Goodglass H, Kaplan E, Barresi B: The Assessment of Aphasia and Related Disorders, 3rd ed. Philadelphia, Lippincott, Williams, & Wilkins, 2001Google Scholar
41. Kaufman AS, Kaufman NL: Kaufman Adolescent and Adult Intelligence Test. Circle Pines, Minn, American Guidance Service, 1993Google Scholar
42. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994Google Scholar
43. Wegesin DJ, Jacobs DM, Zubin NR, et al: Source memory and encoding strategy in normal aging. J Clin Exp Neuropsychol 2000; 22:455–464Google Scholar
44. Chapman LJ, Chapman JP: Problems in the measurement of cognitive deficits. Psychol Bull 1973; 79:380–385Google Scholar
45. Troyer AK, Winocur G, Craik FI, et al: Source memory and divided attention: reciprocal costs to primary and secondary tasks. Neuropsychology 1999; 13:467–474Google Scholar
46. Hinkin CH, Castellon SA, Hardy DJ: Dual task performance in HIV-1 infection. J Clin Exp Neuropsychol 2000; 22:16–24Google Scholar
47. Glisky EL, Rubin SR, Davidson PS: Source memory in older adults: an encoding or retrieval problem? J Exp Psychol Learn Mem Cogn 2001; 27:1131–1146Google Scholar
48. Castelo JM, Sherman SJ, Courtney MG, et al: Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology 2006; 66:1688–1695Google Scholar
49. Moore DJ, Masliah E, Rippeth JD, et al: Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 2006; 20:879–887Google Scholar
50. McArthur JC: HIV dementia: an evolving disease. J Neuroimmunol 2004; 157:3–10Google Scholar



