Neural Correlates of Anxiety in Healthy Volunteers: A Voxel-Based Morphometry Study
Some researchers have argued that normal anxiety and anxiety disorders lie on a continuum where the boundary between normal and pathologic is arbitrary, as various cognitive biases are typically associated with anxiety in both healthy and clinical populations. 4 Therefore, trait and state anxiety provide a reasonable model for anxiety in human subjects and have been used as such in studies exploring cognition in the presence of anxiety. Previous PET, functional MRI, and magnetic resonance spectroscopy studies have investigated the neurobiology of “physiological” anxiety. 5 – 7 The aim of the present study is to evaluate the neural correlates of trait and state anxiety in a healthy population, focusing on brain regional structural characteristics rather than metabolism and function.
The evidence for a neural circuit of fear and anxiety has been established from animal studies, lesion studies, and functional neuroimaging. 8 A key role is held by the amygdala, involved in recognizing signals of danger and in controlling autonomic and behavioral reaction responses to external threat. The medial prefrontal cortex (PFC) and the hippocampus contribute in modulating amygdalar response; the hippocampus supplies information about previous experience of a potentially threatening stimulus while the medial PFC adjusts amygdala activity once the danger is over or the valence of threatening stimuli has changed. 9 , 10 Lack of adequate feedback along these pathways could cause an abnormal reaction to anxiety-evoking stimuli.
In the present study we applied voxel-based morphometry (VBM) to high-resolution structural MRI images 11 to assess the relationship between gray matter interindividual differences in volume and state-trait anxiety, as measured by the STAI. Although very little is known about the correlation between behavioral anxiety measurements and regional brain volume in healthy individuals, 18 based on previous structural MRI studies of anxiety disorders 12 – 17 our hypothesis was that there is an inverse correlation between morphometry of the neuroanatomical network of anxiety described in animal and clinical studies (including the hippocampus, amygdala, and PFC) and self-reported levels of anxiety.
METHODS
High-resolution structural MRIs were obtained for 30 healthy right-handed participants (16 men, 14 women, mean age 28 years ±5.6 SD). None of our participants had a history of alcohol or drug abuse, neurological or psychiatric disorders, or traumatic brain injury. The study was approved by the institutional review board. All subjects gave written informed consent for participation in the study. The images were acquired on a 3 Tesla GE scanner. High resolution, whole-brain T1-weighted spoiled gradient-echo datasets were acquired in the axial plane (TR/TE/Prep Time =8.9/minimum/300; 124 contiguous slices, FOV =24×24, slice thickness =1.5 mm, matrix =256×192). Complete MRI studies of all participants were obtained and reviewed to exclude brain abnormalities. Self-report state (STAI-S) and trait anxiety (STAI-T) measures were completed by subjects prior to the scanning session. 19
The VBM analysis, a statistical parametric technique characterized by user-independent images processing, was carried out using the optimized procedure in SPM2 (Wellcome Department of Cognitive Neurology, London). 20 The optimized procedure is described in detail in Good et al.’s 20 paper and is summarized here. We created a customized brain template which included all of our subjects in order to reduce scanner-specific biases. The template was created by normalizing the structural MRIs of all of the subjects to the Montreal Neurological Institute template and then creating a mean image. The procedure optimized by Good et al. 20 was followed in order to obtain smoothed modulated normalized gray matter images for each subject. These images were entered into multiple regression analyses with state-trait anxiety measures as covariates of interest in order to determine the brain regions whose volume varied with anxiety measures across participants. In order to examine regional differences, we controlled for global differences by entering measurements of total gray matter volume of each participant, in addition to trait and state anxiety measures, into the multiple regression analyses in SPM2. 20 This step was taken in order to correct for total intracranial volume variability. These analyses reveal brain regions in which the volume varies with anxiety measures across subjects. Although the VBM technique has most frequently been used to explore differences in gray matter volume and density between a patient population and matched comparison subjects, it can be used to assess relationship between gray matter volume and performance or symptom measures. 21
RESULTS
The mean STAI-S score was 29.4 (SD=9.1) and the mean STAI-T score 29.1 (SD=6.83). Results on each scale were not statistically different between genders (STAI-S: two-tailed t test for equality of means, t=0.918, df=28; p=0.367; STAI-T: two-tailed t test for equality of means, t=1.025, df=28, p=0.314) and not different from normative data on healthy subjects. 3 Measures of total gray matter volume were not correlated either with STAI-S (Pearson correlation coefficient, two-tailed, r=−0.204, p=0.280) or with STAI-T (Pearson correlation coefficient, two-tailed, r=−0.135; p=0.477).
The Good et al. 20 optimized procedure was followed in order to obtain gray matter partitions for each subject. The resulting smoothed modulated normalized gray matter images were entered into multiple regression analyses using SPM2 to determine the brain regions whose volume varied with the anxiety measures across the participants. In addition to STAI-T and STAI-S measures, total gray matter volumes were entered into the multiple regression analysis in SPM2, in order to control for global differences in gray matter that could bias the correct interpretation of regional gray matter morphometry, as previously discussed.
A conjunction analysis was performed to detect morphometric changes in gray matter which were correlated with trait and state anxiety measures. The significance threshold for results was set at <0.001 (not corrected for multiple comparisons) and, in order to exclude small clusters, an extent threshold of 50 contiguous voxels was chosen. In VBM a statistical threshold of p<0.001 not corrected for multiple comparisons can be used when an a priori hypothesis has been defined according to Ashburner et al. 22
The multiple regression analysis revealed that in several brain regions gray matter volume had an inverse correlation with anxiety ratings ( Table 1 and Figure 1 ). Significant negative correlations have been observed in the prefrontal cortex, in the dorsolateral PFC bilaterally and in the rostral divisions of anterior cingulate gyrus. In the posterior cortex, gray matter volume in the posterior and restrosplenial cingulate cortex showed a negative correlation with STAI-S and STAI-T. Another cluster displaying an inverse correlation included the left parahippocampal gyrus and the left amygdala.
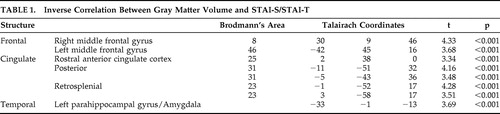 |
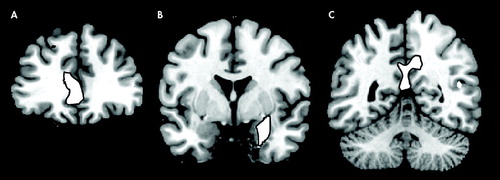
(A) Rostral cingulate (y=38); (B) Left amygdala (y=−1); (C) Posterior cingulate gyrus (y=−52).
In the absence of a well-defined a priori hypothesis, we examined in an exploratory fashion positive correlations between gray matter volumes and STAI-S/STAI-T. Positive correlations were observed only between anxiety scores and regions of the ventrolateral PFC bilaterally ( Table 2 ).
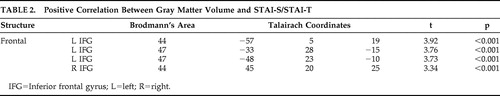 |
DISCUSSION
Our findings show that a distributed neural network, including the parahippocampal-amygdalar regions, the posterior cingulate cortex, and medial and dorsolateral PFC, shows an inverse volumetric correlation with levels of reported anxiety in healthy volunteers. Subjects who had lower gray matter volume in certain areas of the mesial temporal lobe, posterior cingulate cortex, and medial and dorsolateral PFC tended to have higher levels of anxiety as measured using the STAI, while participants who had larger gray matter volume in the same regions had lower levels of anxiety.
A decreased volume of the mesial temporal lobe, including the parahippocampal-amygdalar region is an anatomical hallmark of anxiety disorders. 13 , 14 , 16 , 23 Posttraumatic stress disorder (PTSD) has been associated with reduced hippocampal volumes or function, 12 , 13 , 15 – 17 , 24 and panic disorder has been associated with structural and functional abnormalities in the parahippocampal gyri and amygdala. 25 , 26 Decreased anterior cingulate volume was observed in patients with PTSD using manual tracing volumetric methods and VBM. 27 , 28 Using the technique of VBM, a reduction in left parahippocampal gray matter concentration has been demonstrated in panic disorder patients. 29
The role of the PFC in the expression of fear and anxiety has been previously investigated. Impaired PFC cognitive control over threatening information has been associated with elevated levels of reported and observed anxiety. 32 In addition, the rostral anterior cingulate cortex (ACC) control mechanisms over threat-related distracters may be weakened in anxiety. 6 In the human literature, structural changes in the pre- and subgenual divisions of the ACC have been demonstrated in women affected by PTSD. 33 In animal research, medial PFC has been associated with emotion-related behavior and inhibition of autonomic and defensive reactions. 34 Functional neuroimaging studies have demonstrated an inverse correlation between medial prefrontal cortex activation and PTSD symptom severity. 35 – 37 As a component of a neural network of anxiety, dysfunction of the medial prefrontal cortex would cause perseveration of conditioned fear response even when the threat level has been diminished. 38 Therefore the amygdala is responsible for assessing the emotional valence of stimuli, while the medial PFC adjusts the state of vigilance to the level of threat. 39
The anterior regions of the ACC are involved in the regulation of emotion, while the posterior cingulate cortex (Brodmann’s areas 23 and 31) in the evaluation of the environment, and visuospatial or personal orientation. 38 Despite this dissociation, research studies on animals have shown a strong functional connection between the anterior and posterior cingulate cortices and likely both areas contribute to emotion processing, simple animal phobias and PTSD. 40 , 41 , 42 In addition, the parahippocampal cortex and the posterior cingulate cortex are functionally connected, probably in a circuit devoted to memory and emotion. 41 In the perspective of a neural network of anxiety, the dysfunction of the posterior cingulate gyrus may contribute to some aspects of the cognitive profile observed in trait and clinical anxiety, such as perceptual biases in the interpretation of the environment, and perhaps also the negative memory bias observed in nonclinically anxious individuals.
A PET study has demonstrated an inverse correlation between resting dorsolateral PFC perfusion and state anxiety in older individuals, with a curvilinear relationship between asymmetric perfusion in the dorsolateral PFC and state anxiety. 43 Dorsolateral PFC has well known functional connections with amygdala, parahippocampal gyri, and posterior cingulate, a network of regions devoted to emotion processing. 40 Given the important contribution of dorsolateral PFC in selective attention, 44 we hypothesize that selective attentional biases observed in highly anxious individuals 45 may be associated with structural variability and reduced competence of this region of the prefrontal cortex.
We found that regions of the left ventral and lateral prefrontal cortex (Brodmann’s area 47) displayed positive correlation with STAI-S and STAI-T. Previous studies have demonstrated that these regions, functionally interconnected with the amygdala and inferotemporal regions, are typically activated by emotionally arousing stimuli 40 and are perhaps associated with externally generated emotional states. 46
The reason for a positive correlation between gray matter volume in Brodmann’s area 44 and anxiety scores remains unclear. This finding may reflect the predisposition for the perseverative rumination observed in highly anxious individuals. 47
Our findings support the hypothesis that nonclinical and pathological anxiety lie on a continuum, as they demonstrate analogous neuroanatomical features in addition to their known similar cognitive presentation. Decreased gray matter volume of brain structures involved in the expression of fear and anxiety circuit, including the parahippocampal-amygdalar region, cingulate, and PFC, may lead healthy individuals to have a more pronounced susceptibility to external stressors or physiologic events. The inverse correlation between regional gray matter volume and anxiety suggests that a larger number of neurons and a more extensive intra- and extraregional neuronal network are able to support more efficient functional capabilities, with ultimately lower levels of anxiety in subjects with larger gray matter volume in regions of the mesial temporal and prefrontal cortices. The cause of the mesial temporal and prefrontal gray matter volumetric variability seen in healthy individuals remains unknown and there are two possible explanations for it. Similarly to what is seen in patients affected by anxiety disorders, exposure to corticosteroids during stressful situations may cause modest structural gray matter changes in the amygdalar or hippocampal regions of participants without clinical anxiety disorders where corticoid receptors have been proved to be numerous; alternatively, temporo-mesial volume variability may preexist stressors due to genetic or developmental causes and predispose individuals to anxiety states. 30
The literature suggests a correlation between anxiety and hippocampal volume; however in this study we did not find a correlation between anxiety scores and the volume of the hippocampal formation. This may have occurred for the following reasons: (1) possible suboptimal hippocampal segmentation using VBM given the absence of a sharp macroscopic limit between gray matter and white matter in this region, resulting in a lack of sensitivity to subtle volumetric changes of the hippocampus; 11 , 28 (2) limitations due to our choice of anxiety scale; or (3) hippocampal volumetric changes may be specific to clinical anxiety. Massana et al. 29 reported lower bilateral amygdala volumes in patients affected by panic disorder than in comparison subjects. Nevertheless, in the present study, only the left amygdala volume displayed an inverse correlation with anxiety measures. The finding of a unilateral amygdala correlation may be consistent with observations of a different role in emotion processing for the left and right amygdala. 31
The main strength of our work is the use of an automated volumetric technique to evaluate the association between brain morphometry and self-report scores from behavioral anxiety measures. Nevertheless our study has several limitations. First, we did not compare the results of the VBM analysis with other volumetric MRI techniques. VBM has the advantage of being a fully automated whole brain technique that detects regionally specific gray matter volume differences on a voxel by voxel basis, while commonly used manual or semiautomated volumetric MRI techniques are limited by being operator-dependent and subject to inter- and intraoperator variability. Therefore we believe that VBM is a more suitable tool for detecting subtle structural brain changes in healthy subjects than conventional volumetric MRI technique. Second, our study population included young men and women. There is evidence that age and gender may represent a bias in the volumetric analysis of amygdala and hippocampus in humans. Total gray matter volume was included in the analysis as a confounding variable in order to eliminate global differences in gray matter that may have been due to factors such as age or gender, while allowing the evaluation of regional effects that were due to anxiety levels. Nevertheless, future investigations conducted on samples of healthy men and women separately may be helpful to confirm these findings or clarify gender differences. A recent report shows that there may be an inverse correlation in both genders between right hippocampal volume and higher anxiety-related personality trait, measured using the harm avoidance score from the Temperament and Character Inventory, whereas anterior prefrontal volume correlates with anxiety trait only in women. 18
In the present study each participant underwent only one MRI study. In the future, gray matter volumetric changes and their relationship to anxiety ratings could also be evaluated longitudinally in healthy individuals. These longitudinal studies could help to establish whether persistently high levels of anxiety induce a gradual decrease of gray matter volume in certain regions. A longitudinal VBM analysis could reveal that certain healthy individuals who have relatively high levels of anxiety, but who do not meet the clinical criteria for anxiety disorders, may have consistently lower gray matter volume over time in certain brain regions than age-matched individuals with lower levels of anxiety. This analysis could provide further insights into the plasticity of the anatomical substrate of nonclinical and clinical anxiety.
In summary we found that in normal healthy individuals, regional gray matter volume of brain structures involved in the development of anxiety disorders are inversely correlated with self-reported anxiety measures. Our study suggests that volumetric variability of these brain regions may have an association with the development of an anxious personality trait. The causes of this structural variability, its exact relationship to function, and the temporal evolution of these volumetric findings remain unknown and are topics for future research studies.
1. Lang PJ: The cognitive psychophysiology of emotion: fear and anxiety, in Anxiety and the Anxiety Disorders. Edited by Tuma AH, Maser JD. Hillsdale, NJ, Erlbaum, 1985, pp 131–170Google Scholar
2. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Washington, DC, American Psychiatric Association, 1994Google Scholar
3. Spielberger CD, Gorsuch RL, Lusbene R, et al: Manual for State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychology Press, 1983Google Scholar
4. Davidson RJ: Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 2002; 51:68–80Google Scholar
5. Bishop S, Duncan J, Brett M, et al: Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci 2004; 7:184–188Google Scholar
6. Javanmard M, Shlik J, Kennedy SH, et al: Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. Biol Psychiatry 1999; 1:872–882Google Scholar
7. Grachev ID, Apkarian AV: Anxiety in healthy humans is associated with orbital frontal chemistry. Mol Psychiatry 2000; 5:482–488Google Scholar
8. LeDoux JE: Emotion circuits in the brain. Annu Rev Neurosci 2000; 23:155–184Google Scholar
9. Calder A, Lawrence AD, Young AW: Neuropsychology of fear and loathing. Nat Rev Neurosci 2001; 2:352–363Google Scholar
10. Baxter MG, Murray EA: The amygdala and reward. Nat Rev Neurosci 2002; 3:563–573Google Scholar
11. Ashburner J, Friston KJ: Voxel-based morphometry: the methods. NeuroImage 2000; 11:805–821Google Scholar
12. Bremner JD, Vythilingam M, Vermetten E, et al: MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003; 160:924–932Google Scholar
13. Bremner JD, Randall P, Scott TM, et al: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152:973–981Google Scholar
14. Fontaine R, Breton G, Dery R, et al: Temporal lobe abnormalities in panic disorder: an MRI study. Biol Psychiatry 1990; 27:304–310Google Scholar
15. Gilbertson MW, Shenton ME, Kiyoto K, et al: Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002; 5:1242–1247Google Scholar
16. Gurvits TV, Shenton ME, Hokama H, et al: Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry 1996; 40:1091–1099Google Scholar
17. Villarreal G, Hamilton DA, Petropoulos H, et al: Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry 2002; 52:119–125Google Scholar
18. Yamasue H, Abe O, Suga M, et al: Gender-Common and -Specific Neuroanatomical Basis of Human Anxiety-Related Personality Traits. Cereb Cortex 2008; 18:46–52Google Scholar
19. Spielberger CD, Gorsuch RL, Lushene R, et al: State-trait anxiety inventory. Palo Alto, Calif, Consulting Psychologists Press, 1968Google Scholar
20. Good CD, Johnsrude IS, Ashburner J, et al: A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 2001; 14:21–36Google Scholar
21. Sluming V, Barrick T, Howard M, et al: Voxel-based morphomatry reveals increased gray matter density in Broca’s area in male symphony orchestra musicians. NeuroImage 2002; 17:1613–1622Google Scholar
22. Ashburner J, Csernansky JG, Davatzikos C, et al: Computer-assisted imaging to assess brain structure in healthy and diseased brains. Lancet Neurol 2003; 2:79–88Google Scholar
23. Bonne O, Brandes, D, Gilboa, D, et al: Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry 2001; 158:1248–1251Google Scholar
24. Wignall EL, Dickson JM, Vaughan P, et al: Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biol Psychiatry 2004; 56:832–836Google Scholar
25. Reiman EM, Raichle ME, Butler FK, et al: A focal brain abnormality in panic disorder, a severe form of anxiety. Nature 1984; 310:683–685Google Scholar
26. Nordahl TE, Semple C, Gross M: Cerebral glucose metabolic differences in patients with panic disorder. Neuropsychopharmacology 1990; 3:261–272Google Scholar
27. Woodward SH, Kaloupek DG, Streeter CC, et al: Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry 2006; 59:582–587Google Scholar
28. Yamasue H, Kasai K, Iwanami A, et al: Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A 2003; 100:9039–9043Google Scholar
29. Massana G, Serra-Grabulosa JM, Salgado-Pineda P, et al: Parahippocampal gray matter density in panic disorder: a voxel-based morphometric study. Am J Psychiatry 2003; 160:566–568Google Scholar
30. Uno H, Tarara R, Else JG, et al: Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci 1989; 9:1705–1711Google Scholar
31. Phelps EA, O’Connor KJ, Gatenby JC, et al: Activation of left amygdala to a cognitive representation of fear. Nat Neurosci 2001; 4:437–441Google Scholar
32. Grafman J, Vance SC, Weingartner H, et al: The effects of lateralized frontal lesions on mood regulation. Brain 1986; 109:1127–1148Google Scholar
33. Rauch SL, Shin LM, Segal E, et al: Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport 2003; 14:913–916Google Scholar
34. Phillips ML, Drevets WC, Rauch SL, et al: Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry 2003; 54:501–514Google Scholar
35. Williams LM, Kemp AH, Felmingham K, et al: Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage 2006; 29:347–357Google Scholar
36. Shin LM, Orr SP, Carson MA, et al: Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 2004; 61:168–176Google Scholar
37. Shin LM, Wright CI, Cannistraro PA, et al: A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 2005; 62:273–281Google Scholar
38. Vogt BA, Berger GR, Derbyshire SW: Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci 2003; 18:3134–3144Google Scholar
39. Morgan AM, Le Doux JE: Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 1995; 109:681–688Google Scholar
40. Maddock RJ, Buonocore MH, Kile SJ, et al: Brain regions showing increased activation by threat-related words in panic disorder. Neuroreport 2003; 14:325–328Google Scholar
41. Maddock RJ, Garrett AS, Buonocore MH: Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 2003; 18:30–41Google Scholar
42. Bremner JD, Narayan M, Staib LH, et al: Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 1999; 156:1787–1795Google Scholar
43. Tankard CF, Waldstein SR, Siegel EL, et al: Cerebral blood flow and anxiety in older men: an analysis of resting anterior asymmetry and prefrontal regions. Brain Cogn 2003; 52:70–78Google Scholar
44. Fuster JM: The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe, 3rd ed. New York, Raven Press, 1997, pp 172–174Google Scholar
45. Eysenck MW: Anxiety: The Cognitive Perspective. Hove, UK, Lawrence Erlbaum Associates Ltd, 1992, pp 51–77Google Scholar
46. Yamasaki H, LaBar KS, McCarthy G: Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A 2002; 99:11447–11451Google Scholar
47. Rickels K, Rynn M: Overview and clinical presentation of generalized anxiety disorder. Psychiatr Clin North Am 2001; 24:1–17Google Scholar



