Anxiety Affects Cognition Differently in Healthy Apolipoprotein E ε4 Homozygotes and Non-Carriers
Abstract
It is not known whether apolipoprotein E ε4—an Alzheimer disease susceptibility gene—influences the effects of state-anxiety on executive functioning in healthy individuals. In a prospective cohort study of 185 cognitively normal individuals, there were ε4 homozygotes, heterozygotes, and non-carriers, who did not differ in age, sex, years of education, cognitive test scores, psychotropic medications, and state- or trait-anxiety. However, higher anxiety was associated with significantly worse Trails B performance in the ε4 homozygotes, as compared with ε4 non-carriers. The association of executive-functioning difficulties and anxiety appears more likely to occur in persons who are most at risk for subsequent cognitive decline.
Executive functioning is known to decline with normal aging.1–4 However, studies in elderly individuals with normal cognition found that poorer performance on the Trail-Making Test, Part B (Trails B)—a test of executive functioning—is also predictive of the development of Alzheimer's disease (AD).5,6 Even though memory impairment is the typical hallmark of AD, impairment on tests of executive functioning has been shown to occur early in disease among AD subjects,7 and the pattern of executive-functioning test correlations with resting-state brain glucose metabolism (CMRglc) as measured with positron emission tomography (PET), varies according to disease severity.8 Among cognitively normal individuals, elderly apolipoprotein E (APOE) ε4 carriers exhibited more errors than ε4 non-carriers (NC) in the inhibition/switching condition of a modified Stroop test,9 and cognitively normal APOE ε4 carriers have been shown to have more difficulty with divided attention than matched ε4 non-carriers.10 The APOE ε4 allele is a well-established AD susceptibility gene.11 Studies of mice without APOE ε4, compared to wild-type mice on measures of anxiety and dexamethasone suppression imply that APOE ε4 also plays a role in the regulation of anxiety.12 An earlier study also reported, consistent with the mice data, that, among human AD patients, APOE ε4 homozygotes exhibited higher anxiety scores than ε4 non-carriers.13
Anxiety may impair attentional control14 by altering prefrontal activity.15 Middle-aged, cognitively normal, APOE ε4-homozygote (HMZ) individuals were previously found to have reduced prefrontal metabolism relative to matched NCs,16 suggesting that executive functioning may be differently affected in HMZ, as compared with NCs, by states that alter prefrontal functioning; that is, anxiety states. In an earlier exploratory study, we found a significantly greater correlation between higher chronic anxiety symptoms as measured by the Personality Assessment Inventory and poorer performance on the Wisconsin Card-Sorting Test (WCST), a measure of problem-solving, in middle-aged, cognitively normal HMZs than NCs.17
In this study, we set out to replicate our earlier findings in an independent sample and, in addition to trait-anxiety, examine the effects of state-anxiety on executive functioning in cognitively normal HMZ ε4 heterozygotes (HTZ) and NCs.
METHOD
Anxiety and Neuropsychological Measures
The Spielberger State–Trait Anxiety Inventory (STAI)18 was chosen as a well validated and widely-used measure of state- and trait-anxiety. The State-Anxiety scale (STAI–Y1) is a 20-item self-report questionnaire that evaluates current feelings of apprehension, tension, nervousness, and worry; the Trait-Anxiety scale (STAI–Y2), similarly, screens for persistent anxiety traits. We added a visual-analog anxiety scale to provide a separate measure of state-anxiety that focused just on physiologic manifestations of anxiety. Subjects were asked to place a mark on a bar indicating their current level of nervousness and tension, difficulty relaxing, fatigue, sweaty palms, tremulousness, irregular heartbeats, and/or shortness of breath. They completed anxiety measures immediately before and after neuropsychological testing, but our primary outcome measures were the pre-testing anxiety measures.
We chose the Trails B score of the Trailmaking Test1 as the primary measure of executive functioning, given the evidence in the literature,5,6,14 and reasoning that a timed test of set-shifting would be more sensitive to the effects of state-anxiety than an untimed test of problem-solving. The Trailmaking Test is sensitive to prefrontal cortical functioning.1 It is given in two parts, A and B, and subjects are urged to complete the tasks as quickly as possible without lifting the pencil from the paper. In Part A, subjects draw lines to connect consecutively-numbered circles. In the Trails-B, subjects must connect the same number of circles, alternating between numbers and letters. The examiner points out errors as they occur, and the score is derived from the number of seconds required to complete the task without errors.
The Wisconsin Card-Sorting Test assesses problem-solving by asking subjects to determine which criteria are needed to sort a deck of cards. Subjects are told only whether each response is right or wrong and are never told the correct sorting principle. After a predetermined number of successful trials, the rules change, and the problem-solving task begins again, up to a maximum of six cycles. Separate scores are given for total categories completed, total errors made, and the total number of perseverative errors made.
Both the Trailmaking Test and the Wisconsin Card-Sorting Test were given as part of a larger neuropsychological evaluation requiring approximately 4 hours for completion. Neuropsychological testing was supervised by a neuropsychologist and administered by a trained psychometrist blinded to the purposes of the anxiety questionnaires, the hypotheses of this study, or the participants' APOE status.
Study Population
Subjects age 50 to 66 years (19 HMZ, 59 HTZ, and 107 NC), were consecutively drawn from an ongoing longitudinal study of aging in cognitively normal adults,19 whose neuropsychological testing was performed between 2005 and 2008. Subjects were generally healthy, with no unstable medical or psychiatric conditions, a Mini-Mental State Exam score of ≥27, and no cognitive complaints. Genetic determination of APOE genotype was done using a polymerase chain reaction-based assay,20 for which participants understood that they would not receive results. All individuals gave their written, informed consent, approved by the Mayo Clinic Institutional Review Board, to participate in this study.
Data Analysis
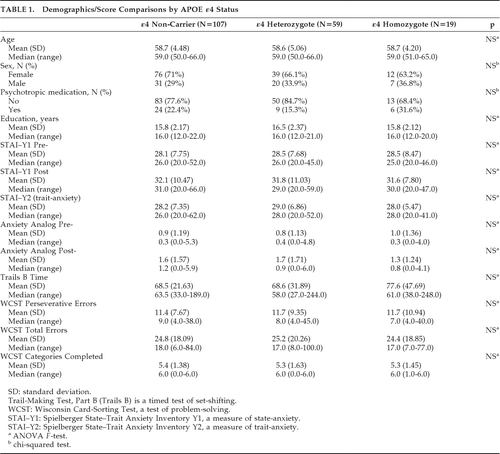
HMZ, HTZ, and NC characteristics were compared by ANOVA F values for continuous values and chi-square tests for categorical variables. The change in a given executive-functioning measure per point-increase in a given anxiety measure (i.e., slope) of HMZ and of HTZ, were compared with that of NCs by using a general linear model with terms for anxiety, genotype, and anxiety x genotype interaction. The interaction terms indicated the difference between the slope for HMZ and that of NC and the difference between the slope for the HTZ group and that of NC.
RESULTS
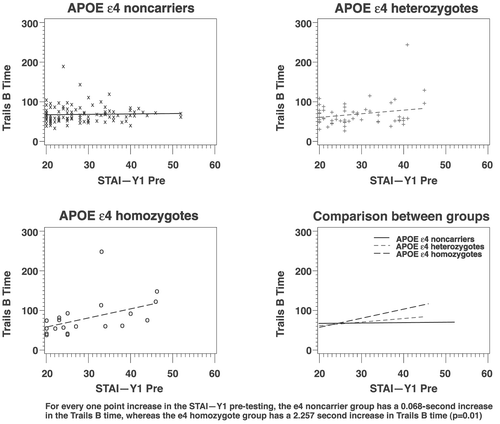
HMZ, HTZ, and NC groups did not differ in age, sex, years of education, cognitive test scores, use of psychotropic medications, and state- or trait-anxiety (Table 1). The demographic description and neuropsychological testing of the subsets parallel those of the entire study population. All general-linear modeling results appear in Table 2. The estimated increase in Trails B time per point-increase in state anxiety, either before or after testing, for HMZs was significantly higher than NCs' (p=0.01 with pretesting STAI; p<0.001 with pretesting visual-analog scale; p<0.001 with post-testing STAI; p<0.001 with post-testing visual-analog scale; Table 2). Graphically, this is depicted in Figure 1 as a difference in slope when comparing HMZ with the NC group.
 |
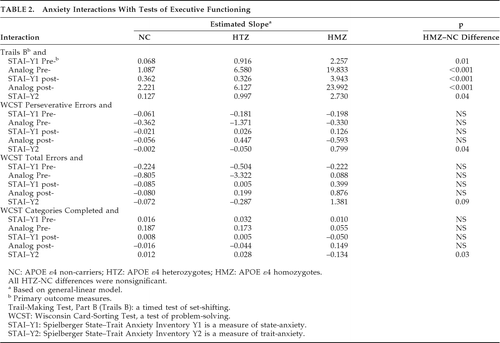 |
There was a significant effect of trait anxiety on HMZ performance, as compared with NC, for WCST perseverative errors (p=0.04), WCST categories completed (p=0.03), Trails B (p=0.04), and a similar trend with the WCST total errors (p=0.09).
All differences in slope when comparing HTZ to NC were not statistically significant. However, there remained a significant effect of pretesting state anxiety on the combined group of HTZ and HMZ, that is, carriers′, performance on Trails B compared with NCs (p=0.03 for pretest STAI–Y1; p=0.006 for pretest visual-analog scale; p=0.02 for the post-test visual-analog scale; post-test STAI–Y1 was not statistically significant).
DISCUSSION
This study replicates our previous findings of an effect of anxiety symptoms on test scores in an independent sample and extends those results by finding an additional effect of state-anxiety on a timed test of executive functioning in HMZs versus NCs. Despite similar anxiety levels in the two groups overall, the effect of anxiety during testing is notably different for HMZs as compared with NCs. We were unable to demonstrate a significant difference from NC in the HTZ, but, for the primary outcome measures, the combined group of HMZs and HTZs also showed an effect of state-anxiety as compared with NCs. It is possible that the smaller number of HMZs allowed for a misleadingly larger effect from a few outliers that may have disappeared with larger numbers. However, we conducted sensitivity analyses in which three subjects with Trails B times greater than 150 sec (one NC, one HTZ, and one HMZ) were removed. Slopes when modeling Trails B time x state- and trait-anxiety measures remained statistically significantly different between HMZs and NCs for pretest STAI (p=0.01), post-test STAI (p=0.02), pretest visual-analog scale (p=0.03), and trait-anxiety (p=0.009), although the estimated slopes were somewhat lessened by the removal of the potentially outlying Trails B time in the HMZ group. A difference in slopes between HMZ and NC for the post-test visual-analog scale did not reach statistical significance (p=0.15). Also, the first study13 had 42 individuals (no overlap with the current study) in each group, and the effect in HMZs was similar to that observed in the current study.
Because the exploratory first study did not reveal an interaction of anxiety and APOE status on measures of memory, we limited our hypothesis and subsequent investigation to measures of executive functioning. However, it is possible that anxiety may negatively affect performance on any cognitive task that places high demands on selective attention, and future studies should investigate whether there is a differential interaction with genotype in other cognitive domains.
A limitation of this study is that we did not experimentally induce anxiety, and the range of anxiety levels was relatively low. Given that our subjects were psychiatrically healthy, one should use caution in applying our conclusions to patients with pathological anxiety. Indeed, if the subjects had anxiety disorder, we might well expect an effect of anxiety on cognition in the NC group. On the other hand, we would predict that a similarly-anxious HMZ group would show an even greater effect.
A small number of subjects were taking psychotropic medication, which could have affected the results. Although there was a similar percentage using psychotropic medication in each group, because of the small numbers, we did not examine whether there was a differential effect of medication in HMZs. Likewise, because the study was not powered for gender subsets, we did not examine whether the effects of anxiety might differ in women and men, especially for HTZs.
This study implies that stress and anxiety may induce executive-functioning difficulties during everyday functioning or on cognitive testing disproportionately among those most at risk for developing AD. We know that, as compared with HTZs and NCs, HMZs are most at risk for subsequent cognitive decline. We will have to continue to follow these subjects longitudinally in order to verify whether the individual subjects with the largest effect of anxiety on executive functioning subsequently exhibit cognitive decline. Nonetheless, clinicians might want to consider repeat testing in 6 to 12 months for a patient exhibiting impaired performance on neuropsychological testing due to nervousness, particularly when the patient does not have an anxiety disorder.
1. : Neuropsychological Assessment, 4th Edition. New York, Oxford University Press, 2004Google Scholar
2. : Differential course of executive-control changes during normal aging. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2007; 14:370–393Crossref, Medline, Google Scholar
3. : Aging, executive control, and attention: a review of meta-analyses. Neurosci Biobehav Rev 2002; 26:849–857Crossref, Medline, Google Scholar
4. : An application of prefrontal cortex function theory to cognitive aging. Psychol Bull 1996; 120:272–292Crossref, Medline, Google Scholar
5. : Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc 2001; 7:631–639Crossref, Medline, Google Scholar
6. : Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology 2000; 55:1847–1853Crossref, Medline, Google Scholar
7. : Attention and executive deficits in Alzheimer's disease: a critical review. Brain 1999; 122( Pt 3):383–404Crossref, Medline, Google Scholar
8. : Metabolic correlates of executive dysfunction: different patterns in mild and very mild Alzheimer's disease. J Neurol 2007; 254:1052–1065Crossref, Medline, Google Scholar
9. : Deficits in inhibition and flexibility are associated with the APOE ε4 allele in nondemented older adults. J Clin Exp Neuropsychol 2005; 27:943–952Crossref, Medline, Google Scholar
10. : Working memory and apolipoprotein E: what's the connection? Neuropsychologia 2002; 40:2226–2233Crossref, Medline, Google Scholar
11. : Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer's disease. Neurology 1993; 43:1467–1472Crossref, Medline, Google Scholar
12. : Role of apolipoprotein-E in anxiety. Neural Plast 2007; 2007:91236Crossref, Medline, Google Scholar
13. : APOE isoforms and measures of anxiety in probable AD patients and APOE -/- mice. Neurobiol Aging 2005; 26:637–643Crossref, Medline, Google Scholar
14. : Anxiety and cognitive performance: attentional control theory. Emotion 2007; 7:336–353Crossref, Medline, Google Scholar
15. : Neural correlates of anxiety in healthy volunteers: a voxel-based morphometry study. J Neuropsychiatry Clin Neurosci 2009; 21:199–205Link, Google Scholar
16. : Preclinical evidence of Alzheimer's disease in persons homozygous for the ε4 allele for apolipoprotein E. N Engl J Med 1996; 334:752–758Crossref, Medline, Google Scholar
17. : A distinctive interaction between chronic anxiety and problem-solving in asymptomatic APOE ε4 homozygotes. J Neuropsychiatry Clin Neurosci 2004; 16:320–329Link, Google Scholar
18. : Manual for the State–Trait Anxiety Inventory (Form Y). Palo Alto, CA, Consulting Psychologists Press, Inc, 1983Google Scholar
19. : Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE ε4 allele. Neurology 2004; 62:1990–1995Crossref, Medline, Google Scholar
20. : Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HHAI. J Lipid Res 1990; 31:545–548Medline, Google Scholar



