Treatment of Resistant Obsessive-Compulsive Disorder With Ventral Capsular/Ventral Striatal Gamma Capsulotomy: A Pilot Prospective Study
A smaller double-shot, bilateral gamma knife anterior capsulotomy lesion has been recently proposed at Brown University, with few side effects and good efficacy profile, named (VC/VS) gamma capsulotomy. 8 Preliminary data indicated that clinical response (at least 35% reduction in Yale-Brown Obsessive Compulsive Scale [Y-BOCS] scores) was obtained in 13 out of 22 patients (59%) after 1 year and in 15 patients (69%) after 2 years of follow-up. 8
In this article we report preliminary findings from five patients who underwent VC/VS gamma capsulotomy for refractory OCD with a minimum follow-up period of 36 months. The aims of this study were to report preliminary findings of efficacy of the technique and to assess the safety of this procedure, including general medical and neurological adverse events/complications.
MATERIALS AND METHODS
Ethical Issues
This study complies with the guidelines for neurosurgery of severe psychiatric disorders in Brazil 9 and was approved by the Brazilian National Commission of Research Ethics (CONEP). Approval was also granted by local Ethics Committees at the University of São Paulo Medical School and Santa Paula Hospital, respectively, the institutions where the patients were clinically assessed and submitted to radiosurgery.
Written informed consent was obtained, with a member from the Brazilian OCD Patients Foundation present to ensure adequate protocol understanding from all patients. An independent panel composed of two psychiatrists appointed by the Regional Medical Council of São Paulo and the president of the Brazilian OCD Patients Foundation reviewed video-taped interviews confirming the patients’ adequate knowledge of benefits and risks related to gamma radiosurgery.
The research team will provide all patients with systematic assessments of adverse events and complications for a minimum follow-up period of 5 years.
Clinical Assessments
Patients included in the study were submitted to a detailed psychiatric history, as well as physical, neurological, and psychiatric examinations. Psychiatric axis I and axis II comorbidities were further assessed by the Structured Clinical Interview for DSM-IV (SCID) and the Structured Interview for DSM-IV Personality Disorders (SIDP-IV). 10 , 11
Sample Selection
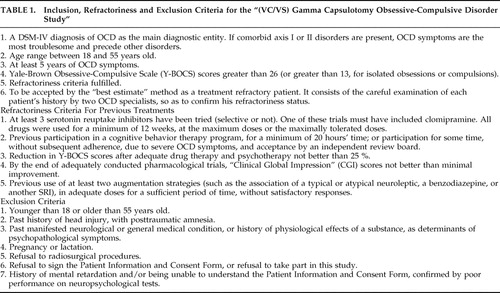
Thirteen severe OCD patients from various States in Brazil were initially screened as candidates for surgery. Only five patients (38.5%) fulfilled our inclusion and refractoriness criteria (see details in Table 1 ). The main reasons for exclusion were lack of adequate medication/CBT trials (four patients), a history of full treatment response after the introduction of a previous medication (one patient) and other main axis I/II diagnosis (three patients), namely factitious disorder, severe bipolar disorder and one case of prominent paranoid personality disorder.
 |
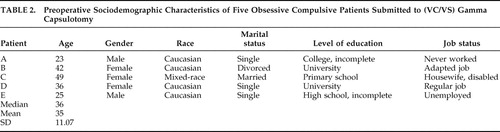
The five patients included (two men, three women, mean age 35.0±11.0 years, mostly single) had a median of 18 years of severe, disabling OCD symptoms and considerable psychosocial impairment. Three patients (A, C, E) were unable to complete their University degrees. Only one patient (D) was working regularly at the time of surgery ( Table 2 ).
 |
All patients reported early onset of their obsessive-compulsive symptoms (before 10 years of age). Compulsions appeared first (median age for first compulsions was 8 years) ( Table 3 ). Obsessive-compulsive symptoms important enough to interfere with daily living activities were present, on average, by the age of 18.
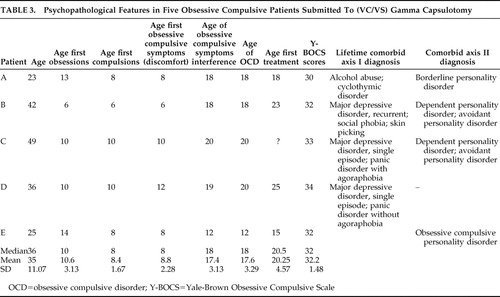 |
Major depressive and panic disorders were the most prevalent axis I comorbidities ( Table 3 ). Cluster C personality disorders such as dependent and avoidant personality disorders (patients B and C) and obsessive-compulsive personality disorder (patient E) were common ( Table 3 ). There were no comorbid tic disorders.
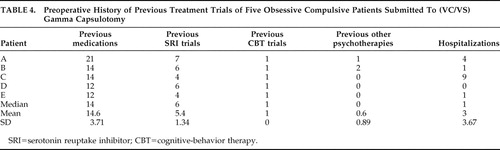
Specific treatments were usually sought at a median age of 20.5 years, or 2.5 median years after full-blown OCD. The mean number of previous medication trials was 14.6—more specifically, 5.4 different SRI trials, one of which was with clomipramine ( Table 4 ). All patients had received adequate CBT treatment, except for patient E, who tolerated only very few sessions. Patients were told to maintain their medications unchanged after surgery. However, prescription modifications were necessary in two patients (C and E), and one of the responders independently reduced his dosage (A).
 |
Follow-up Assessments
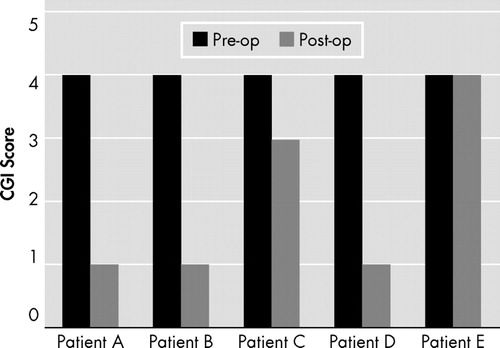
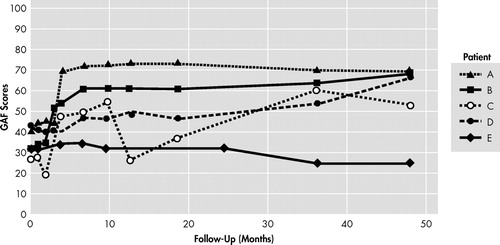
Clinical global changes were prospectively assessed by the Clinical Global Impression (CGI) Scale and Global Assessment of Functioning (GAF) Scale. 12 , 13 The presence and severity of OC symptoms and tics were respectively determined by the Y-BOCS and the Yale Global Tic Severity Scale. 14 – 16 The Beck Depression Inventory (BDI) and the Beck Anxiety Inventory (BAI) were administered to all patients. 17 , 18 All inventories were applied before surgery and at every subsequent follow-up visit. Patients who missed their follow-up appointments were interviewed by phone to minimize loss of data collection.
Sleep and cognitive, emotional and/or physical adverse events were prospectively assessed by a detailed questionnaire (SAFTEE Event List and Interview). 19
Postoperative follow-up visits were scheduled at 2 weeks, 1 month, 2 months, and subsequently at 3, 6, 9, 12, 24, and 36 months after surgery. At 48 months of follow-up, two patients (B and D) completed a full follow-up visit, whereas three patients (A, C, and E) were interviewed by telephone.
Clinical Response Criteria
Response criteria were defined as a minimum of 35% reduction in Y-BOCS scores and an “improved” or “much improved” score on the CGI.
Operative Technique
A preoperative stereotactic MRI was obtained, and determination of dose planning and target selection was performed using a dedicated software (Leksell GammaPlan, 5.32, 2002).
The final step of the procedure was the exposure of the targets with the patient in the Leksell Gamma Knife model B. Bilateral, double-shot capsulotomy lesions were targeted at the ventral portions of the anterior limb of the internal capsule, approximately 10 mm rostral to the anterior commissure. The target at the anterior limb of the internal capsule was covered by a 50% isodose line; maximum dose at 100% was 180 Gy. The bilateral targets were each exposed by two focus positions (“shots”) from the converging sharply collimated beams of gamma radiation from 201 60 Co sources, using 4 mm collimators. Sensitive structures such as the optic nerves and lenses were protected, maintaining radiation below a safe threshold in these regions. The mean total time of exposure to radiation was 480 minutes (or 240 minutes for each hemisphere). Radiation rate from the 60 Co sources was 1.788 Gy/min.
The American (BG and GN) and the Brazilian team (ACL, MC, ECM) worked together to determine optimal target localization and dose planning for all patients, either in person (cases A, B and C) or through electronic communication (cases D and E). Agreement from all members of the team was achieved previous to each procedure.
MRI Acquisition
Structural MRI scans were acquired for all five OCD patients pre- and postoperatively (range 9 to 24 months) using a 1.5 Tesla Signa LX CVi scanner (version 9.1, 2004, General Electric, Milwaukee, Wisconsin).
Statistical Analysis
All variables were summarized using descriptive statistics (frequency, median, mean and SD). Small sample sizes and noncontinuous variables precluded parametric or nonparametric statistical analysis.
RESULTS
Assessment of Efficacy of the Procedure
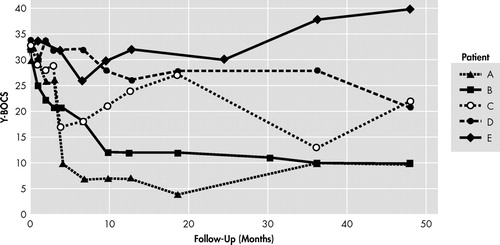
Among the five patients submitted to gamma-capsulotomy, patients A and B (40%) fulfilled our response criteria after 12 months, and three patients (60%), including patient D, fulfilled criteria after 48 months of follow-up. Y-BOCS reductions ranged from 38% to 69% ( Table 5 , Figure 1 , and Figure 2 ) among responders. Mean Y-BOCS scores changed from 32.2±0.67 (standard error of mean [SEM]) to 20.6 ± 5.49 (SEM) after 48 months of follow-up. GAF improvements also reflected these changes ( Figure 3 ). Patient C met our response criteria until 9 months of postoperative follow-up, with a subsequent relapse; she again became a responder when 36 months of follow-up was reached, but relapsed 4 years after surgery. Patient D showed only 18% reduction of the Y-BOCS at the 36th month of follow-up, but became a full responder (38% reduction of the Y-BOCS) by 48 months of follow-up. On the other hand, patient E’s OCD symptoms deteriorated after 24 months of follow-up (25% increase on the Y-BOCS).
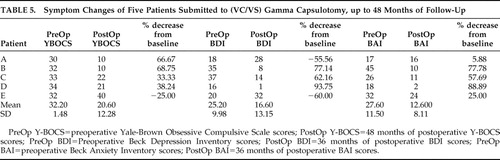 |
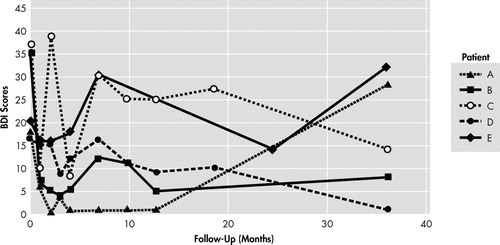
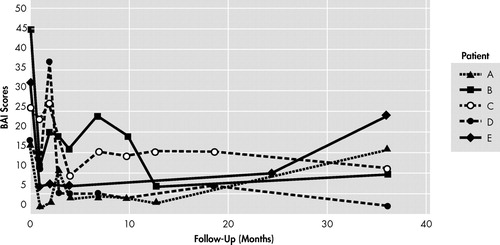
Beck Depression Inventory scores oscillated considerably along follow-up visits. Nevertheless, full and partial responders tended to present important reductions of the BDI scores, except for patient A, who developed a relapse of depressive symptoms at 36 months of follow-up (56% increase of scores from baseline) ( Table 5 , Figure 4 ). Patient E, a nonresponder, also showed further impairment (60% increase of BDI scores from baseline). A similar pattern was found with BAI scores, with more robust anxiety decrements (mainly with patients B, C and D) ( Table 5 , Figure 5 ).
Three patients had their medications changed during the study. Patient A did not tolerate higher dosages of antidepressants. He spontaneously changed his medication, from fluvoxamine, 100 mg/day, venlafaxine, 150 mg/day, quetiapine, 100 mg/day and clonazepam, 2 mg/day, to fluvoxamine, 50 to 100 mg/day, quetiapine, 25 mg/day, and clonazepam, 2 mg/day. For patient C, clomipramine dosage was increased from 225 mg/day to 300 mg/day, plus risperidone, 4 mg/day, and diazepam, 10 mg/day, during the study, due to depressive symptoms. By 36 months of follow-up, she was no longer taking risperidone, but OCD symptoms had improved. Patient D was initially taking citalopram, 60 mg/day, plus olanzapine, 2.5 mg/day, with no subsequent improvements on OCD. It was changed to fluoxetine, 40 mg/day, plus ziprasidone, 40 mg/day.
Assessment of General Medical and Neurological Adverse Events
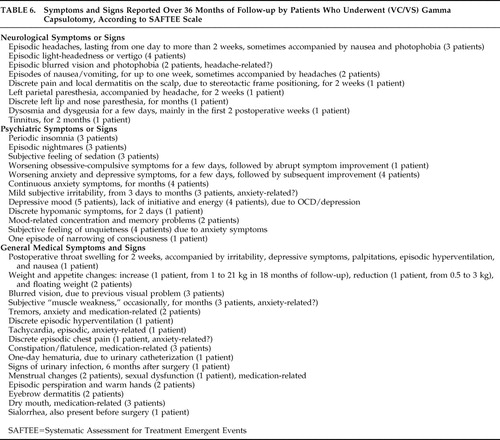
Table 6 describes adverse events reported by patients, according to the Systematic Assessment for Treatment Emergent Events (SAFTEE) scale. Headaches (lasting days to weeks), light-headedness/vertigo, weight changes and episodic nausea/vomiting were observed. Headaches were usually responsive to nonsteroidal anti-inflammatory drugs, except for patient C, who required steroids for a few weeks. Another patient required bladder catheterization during the surgical procedure, presenting hematuria that resolved after 1 day (patient D).
 |
MRI Assessments
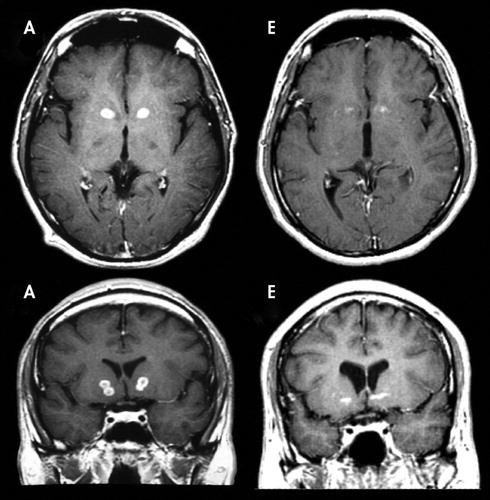
Post operative MRI scans showed that all radiosurgical targets located exactly where planned preoperatively ( Figure 6 ). All patients, with the exception of patient E, showed postoperative bilateral double-shot anterior capsulotomy lesions, indicated by hypointense rounded volumes on T1-weighted, T2-weighted and FLAIR images, with hyperintense borders on FLAIR and T2-weighted images and peripheral ring enhancement following paramagnetic contrast media administration, adjacent to the head of the caudate nuclei. Patient E, however, showed only minimal lesions in target areas, especially in the left hemisphere, as indicated in most image series ( Figure 6 and Figure 7 ).
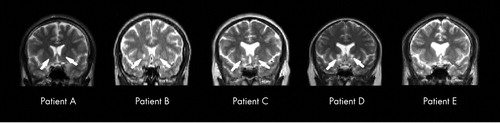
Patient A, who does not show marked lesions in this acquisition, demonstrated clear lesions on other acquisitions (see Fig. 7). This is not the case of patient E, who showed only minimal lesions on the left hemisphere in all acquisitions.
DISCUSSION
Procedure Efficacy
Previous anterior capsulotomy techniques produced a relatively high incidence of side effects, possibly as a consequence of larger lesions. 20 , 21 Aiming to improve results and reduce side effects, smaller and standardized capsulotomy lesions were developed (the VC/VS gamma capsulotomy). 8 This improved procedure is based on a more precise target definition within the internal capsule, employing smaller collimator sizes (4 mm), consequently aimed at producing smaller lesions albeit not compromising efficacy.
Our preliminary findings suggest that this procedure holds promise for selected severe and treatment resistant OCD patients. Among the five patients enrolled in this study, at the end of a 3-year follow-up period, three (60%) presented full response after surgery (67%, 69% and 38% reductions in Y-BOCS severity), and one had a partial response (33%). On the other hand, the one patient who was a nonresponder to surgery also developed worse OCD symptoms in the long-term follow-up, though maintaining his previous psychosocial impairment.
The three patients who improved the most and the partial responder showed markedly increased personal autonomy and reintegration into society, in addition to relief of persistent subjective distress.
Although comparison across studies is limited for methodological reasons (see Greenberg et al. 22 for discussion), clinically significant improvement after larger capsulotomy lesions (including an earlier gamma capsulotomy technique) was previously reported in five out of nine patients (55%) and seven out of 10 patients (70%). 6 , 7 These rates are comparable to ours (3 out of 5 patients or 60%). However, we used more stringent response criteria. Previous studies were less rigorous regarding OC symptom amelioration and did not include measures of global improvements (such as CGI scores). Analyzing data from previous Gamma Knife techniques, but response criteria similar to ours, Rück 21 described response rates similar to ours (five out of eight patients, 62.5%).
The delayed amelioration of symptoms observed in our study could result from multiple sources and even be unrelated to the surgery. However, the latter interpretation is less likely, since OCD in the overwhelming majority of our patients was a chronic illness (mean OCD duration of 17.4 years), unresponsive to multiple conventional treatment schemes.
It remains unclear how gamma capsulotomy lesions result in OCD symptom improvement. One hypothesis is that bilateral lesions of the anterior capsule could alter information flow in thalamo-orbital and thalamo-cingulate neuronal circuitry (particularly the subgenual cingulate), which is related to the pathophysiology of OCD, and thus be involved in the changes in OCD symptoms observed after surgery. Interestingly, functional imaging data from a small cohort of patients with severe anxiety disorders undergoing this type of neurosurgery showed reduced activity within medial orbitofrontal cortex after the intervention. 22 , 23
In addition, edema and necrosis produced by gamma radiation surrounding the targets may compromise the adjacent striatum 24 and nucleus accumbens. Therefore, it is possible that additional cortico-striato-pallido-thalamico-cortical circuits are interrupted, especially those involving the ventral striatal-pallidal system and the extended amygdala. 25 – 27
It is unknown whether unilateral lesions would be as efficacious as bilateral targeting. One previous MRI study of anterior capsulotomy for OCD suggested that appropriate lesioning of the right anterior capsule might be critical to subsequent therapeutic response. 7 It is noteworthy in our study that the only patient who did not show any clinical response to surgery had a minimal left anterior capsule lesion, whereas all the other patients showed clear bilateral lesions.
It is not surprising that most of our patients also reported postoperative reductions in anxiety and depression scores. Anxiety disorders and refractory depression have been successfully treated with anterior capsulotomy by thermolesion. 28 , 29 In our sample, persisting reductions in anxiety scores were more prominent than reductions in depressive symptoms (mean reduction of 15 score points or 54%, versus 8.6 score points or 34%). Patient A presented with worse scores on BDI and BAI in his last follow-up visit, and this may be attributed to his inadequate compliance with medications. He nonetheless maintained low Y-BOCS scores. Patient E, a treatment nonresponder, showed marked worsening of BDI and BAI scores, and OCD symptoms deteriorated. Therefore, it is possible that the improvement in anxiety and depressive symptoms by surgical intervention may contribute to the amelioration of OCD symptoms directly or indirectly, although not explaining the maintenance of low Y-BOCS scores in the postoperative follow-up period.
General Medical and Neurological Adverse Events
According to the SAFTEE scale, most adverse events were transient, not interfering with functioning or requiring treatment or hospitalization. Only headache, in one patient, lasted more than a few weeks. In this only case with persistent headache (around 2 weeks’ duration), no evidence of brain swelling was found on MRI, although the patient was empirically treated with corticosteroids for a few weeks. None of these initial symptoms persisted 36 months after surgery. However, it is still early to draw definite conclusions regarding potential long-term adverse effects.
Some of the symptoms and signs assessed by the SAFTEE scale may be attributed either to the direct effects of radiation, or to the effects of anesthesia and head positioning in the Gamma Knife equipment or even be unrelated to surgery. Headaches, nausea, vertigo, weight changes, postoperative throat swelling, 1 day hematuria, local dermatitis, and discrete pain on the scalp were probably related to the surgical procedure. Even though weight changes were common in four patients, only one patient (C) developed a considerable weight gain. Most side effects were mild and lasted for only a few days. We suppose that other symptoms/signs may or may not have been related to surgery.
A less favorable profile of adverse events was reported in previous radiosurgical studies using larger targets. 21 , 29 Rück 21 reported the results of nine patients with OCD submitted to Gamma Knife capsulotomies. Three to four bilateral isocenters and 4 mm collimators were used for lesions in five patients, delivering more than 200 Gy at the center of the lesion on each side. 21 Frontal lobe complications were evident in two patients, as well as urinary incontinence and seizures. It is unclear, however, if this was due to larger lesion volume, higher doses, and/or different targeting.
Limitations
This pilot study has several limitations. First, it included a small cohort and used an open design. Second, as patients came from regions distant to the study site, some follow-up data were obtained only by telephone interview (especially at 48 months of follow-up). For the same reason, postoperative MRI scans schedule was different among patients (range=9 months to 24 months postsurgery). It was not possible to control for medication changes. Furthermore , patient C presented a quite variable clinical course over the 48 months of follow-up, demanding further assessment to reassure her response condition. Finally, neuropsychological and personality changes associated with this surgical procedure are not provided in this article, but they will be described in other publications.
CONCLUSION
In conclusion, this pilot study supports the view that the VC/VS Gamma Knife capsulotomy for resistant OCD may hold promise for a selected group of patients who have otherwise no therapeutic options. Our findings suggest that this procedure may be effective and well-tolerated overall, particularly when compared to the previous Gamma Knife technique that employed larger lesions. These conclusions, however, must be tentative, given the limitations of the short follow-up period (4 years) and our open study design. So far, only a few pilot trials of neurosurgery for resistant OCD have used a controlled design, all of them with small sample sizes. 30 , 31 Our group is currently conducting the first double-blind, randomized controlled trial on radiosurgery to address this gap in knowledge.
1. Pallanti S, Hollander E, Bienstock C, et al: Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol 2002; 5:181–191Google Scholar
2. Jenike MA, Rauch SL: Managing the patient with treatment-resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry 1994; 55:11–17Google Scholar
3. Perse T: Obsessive-compulsive disorder: a treatment review. J Clin Psychiatry 1988; 49:48–55Google Scholar
4. Rasmussen SA, Eisen JL: Treatment strategies for chronic and refractory obsessive-compulsive disorder. J Clin Psychiatry 1997; 58(suppl 13):9–13Google Scholar
5. Lopes AC, de Mathis ME, Canteras MM, et al: [Update on neurosurgical treatment for obsessive compulsive disorder.] Rev Bras Psiquiatr 2004; 26:62–66 (Portuguese)Google Scholar
6. Rylander G: Försök med gammakapsulotomi vid ångest-och tvångsneuroser. Lakartidningen 1978; 75:547–549Google Scholar
7. Lippitz BE, Mindus P, Meyerson BA, et al: Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: relevance of the right hemisphere. Neurosurgery 1999; 44:452–458Google Scholar
8. Norén G, Lindquist C, Rasmussen SA, et al: Gamma Knife capsulotomy for obsessive-compulsive disorder. American Association of Neurological Surgeons [abstract on the Internet]. 2002. Available at http://www.aans.org//library/article.aspx?ArticleId=12171Google Scholar
9. Miguel EC, Lopes AC, Guertzenstein EZ, et al: Guidelines for neurosurgery of severe psychiatric disorders in brazil: a preliminary proposal. Rev Bras Psiquiatr 2004; 26:7–8Google Scholar
10. First MB, Spitzer RL, Gibbon M, et al: User′s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders – clinician version (SCID-CV). Washington, DC, American Psychiatric Publishing, 1997Google Scholar
11. Pfohl B, Blum N, Zimmerman M: Structured Interview for DSM-IV Personality (SIDP-IV). Washington, DC, American Psychiatric Publishing, 1997Google Scholar
12. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology, Revised (publication ADM 76-338). Rockville, MD, US Department of Health, Education, and Welfare, 1976Google Scholar
13. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text revision. Washington, DC, American Psychiatric Association, 2000Google Scholar
14. Goodman WK, Price LH, Rasmussen SA, et al: The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry 1989a; 46:1006–1011Google Scholar
15. Goodman WK, Price LH, Rasmussen SA, et al: The Yale-Brown Obsessive Compulsive Scale, II: validity. Arch Gen Psychiatry 1989; 46:1012–1016Google Scholar
16. Leckman JF, Riddle MA, Hardin MT: The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989; 28:566–573Google Scholar
17. Beck AT, Ward CH, Mendelson M, et al: An inventory for measuring depression. Arch Gen Psychiatry 1961; 41:561–571Google Scholar
18. Beck AT, Epstein N, Brown G, et al: An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56:893–897Google Scholar
19. Guy W, Wilson WH, Brooking B, et al: Reliability and validity of SAFTEE: preliminary analyses. Psychopharmacol Bull 1986; 22:397–401Google Scholar
20. Kihlström L, Guo WY, Lindquist C, et al: Radiobiology of radiosurgery for refractory anxiety disorders. Neurosurgery 1995; 36:294–302Google Scholar
21. Rück C: Capsulotomy in anxiety disorders. Thesis for doctoral degree (PhD) [Dissertation]. Stockholm, Karolinska Intitutet, 2006Google Scholar
22. Greenberg BD, Price LH, Rauch SL, et al: Neurosurgery for intractable obsessive-compulsive disorder and depression: critical issues. Neurosurg Clin N Am 2003; 14:199–212Google Scholar
23. Mindus P, Ericson K, Greitz T, et al: Regional cerebral glucose metabolism in anxiety disorders studied with positron emission tomography before and after psychosurgical intervention: a preliminary report. Acta Radiol Suppl 1986; 369:444–448Google Scholar
24. Rauch SL: Neuroimaging and neurocircuitry models pertaining to the neurosurgical treatment of psychiatric disorders. Neurosurg Clin N Am 2003; 14:213–223Google Scholar
25. Heimer L: A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry 2003; 160:1726–1739Google Scholar
26. Ribas GC: [Considerations about the nervous system phylogenetic evolution, behavior, and the emergence of consciousness.] Rev Bras Psiquiatr 2006; 28:326–338 (Portuguese)Google Scholar
27. Ribas GC: [Neuroanatomical basis of behavior: history and recent contributions.] Rev Bras Psiquiatr 2007; 29:63–71 (Portuguese)Google Scholar
28. Hurwitz TA, Mandat T, Forster B, et al: Tract identification by novel MRI signal changes following stereotactic anterior capsulotomy. Stereotact Funct Neurosurg 2006; 84:228–235Google Scholar
29. Rück C, Andreewitch S, Flyckt K, et al: Capsulotomy for refractory anxiety disorders: long-term follow-up of 26 patients. Am J Psychiatry 2003; 160:513–521Google Scholar
30. Fodstad H, Strandman E, Karlsson B, et al: Treatment of chronic obsessive compulsive states with stereotactic anterior capsulotomy or cingulotomy. Acta Neurochir (Wien) 1982; 62:1–23Google Scholar
31. Nuttin BJ, Gabriels LA, Cosyns PR, et al: Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery 2003; 52:1263–1272Google Scholar



