Patterns of Cortico-Limbic Activations During Visual Processing of Sad Faces in Depression Patients: A Coordinate-Based Meta-Analysis
Abstract
The author retrieved 10 functional magnetic resonance imaging studies about visual tasks for emotional faces in subjects with depression. The activation foci were then summarized and entered into a coordinate-based meta-analysis. The depression group showed significantly increased activations in the left striatum and left parahippocampal gyrus; the control group showed increased activations in the left medial frontal gyrus, left middle frontal gyrus, right thalamus, left anterior cingulate, and superior frontal gyrus. The study suggests that depression patients have limbic activations, and controls have fronto-thalamic activations with visual processing of emotional faces.
Visual processing of emotional faces is important for the pathophysiology of depression. There is much evidence showing that processing of facial emotions will involve both cortical and subcortical systems.1 Fitzgerald et al.’s meta-analysis concludes that frontal deactivations and subcortical activations might play a role in deficits of emotional processing for depression.2 Among the subcortical system, the striatum is important for the recognition of facial emotions. Non-threatening faces with emotion, such as sad faces, are associated with activations in the left ventral striatum.3 Fu et al. indicated that depressed patients would have heightened capacities for activations in the ventral striatum and amygdala before antidepressant treatment and that therapy would change activities in these subcortical areas.4 Depression is also associated with increased activations in the left parahippocampal gyrus (PHG) and amygdala with experiencing sad faces.5 Delaveau et al. also mentioned that the limbic system, such as PHG and amygdala, might be hyperactive in depression patients due to loss of inhibitory function from the frontal system.6 Suslow et al. reported that thalamic activities are correlated with personality traits in the recognitions of facial expressions of emotions, and they also suggested that the thalamus might form connections between the striatum and frontal system to form the fronto-striato-thalamic circuit in the visual processing of facial emotions.7 Expectations for unpleasant stimuli would also provoke the heightened response in the thalamus and striatum. Dysregulation in subcortical regions has also been hypothesized as the etiological origin of depression.8 These studies suggest that thalamus, amygdala, and PHG might play vital roles for modulating brain activities in the emotion-specific task.9
These subcortical (limbic) regions also interact with the frontal (cortical) system to form a fronto-limbic network for the modulations of emotional faces.3 The frontal (cortical) system usually inhibits excessive reactions of the limbic subcortical system, which is probably important for visual processing of emotional faces. In the frontostriatal model of depression, the superior frontal gyrus (SFG), medial frontal gyrus (MeFG), middle frontal gyrus (MFG) and anterior cingulate cortex (ACC) belong to the frontal system.10 SFG has been reported to be correlated with emotional response to sad faces in alexithymia patients, which is also a neurobiological model for pathophysiology of visual processing of emotions.11 In the stereotactic meta-analysis conducted by Steele et al., they found that healthy controls showed MeFG activation while experiencing sad emotions.12 In the depression model, tryptophan depletion also resulted in reduced MeFG activities associated with loss of empathetic function and loss of adaptive function during threatening context in patients with depression.13 MFG also accounts for the differences of processing happy versus sad faces in brain activities; this area is prone to produce robust responses while experiencing emotional stimulation of social events.14 ACC is usually believed to be associated with sad emotional identification, social behavior, and subjective emotional state.15 The ACC is an important area for processing emotional faces, independently of ages.16,17 ACC is also hypothesized to be the key area for the processing of affective information.18 Davidson et al. also found that depression patients with greater response of the ACC to negative stimuli of emotions showed robust treatment response after antidepressant treatment, which suggested ACC’s role in the prognosis of depression.19 The ACC in the modulation of negative emotions in depression has been replicated in the following studies,18,20,21 and these studies all supported dysregulation of ACC for emotion modulations of patients with depression.
According to the above literature, the modulations of cortical and subcortical systems for the visual processing of emotional stimuli might be disturbed in depression. We hypothesized that we could find abnormal activations in cortical and subcortical systems, such as amygdala, thalamus, striatum, PHG, MeFG, MFG, SFG, or ACC when depression patients received visual stimulation of sad faces. We utilized a meta-analysis using the activation likelihood estimation (ALE) method to explore the patterns of activations in brains of patients with depression and controls upon exposure to emotion-relevant facial stimuli. We also hypothesized that there should be differences between depressed subjects and controls during the visual task of emotional faces.
Methods
Literature Selection and Data Collection
We conducted systematic a search in PubMed, Scopus, and Medline, using the following keywords “depression,” “visual,” “sad faces,” and “magnetic” to find the articles we needed. We also collected data for two different models; one was “depression versus control,” which showed the activated areas for patients with depression when compared with a healthy-control group; another was “control versus depression,” which revealed the brain activations for the controls when compared with a depression group. For each of the searched studies, we extracted the coordinates (x, y, z) for the foci of interest and the corresponding number of subjects. Only overactivation foci reported as significant at a p value <0.05 in the source studies were included. When necessary, a transformation from the Montreal Neurological Institute to the Talairach space was performed, using the icbm2tal algorithm, according to the ALE method, which was implemented in GingerALE software (www.brainmap.org/ale/). We also followed the MOOSE guideline to report the subsequent meta-analysis of the relevant observational studies.22 We found 10 related articles4,5,21,23–29 by the above search method. There were 301 subjects, 43 experiments, 117 conditions, and 298 foci of activations. Within these 10 studies, we established two different groups from each study design and results. In the “depression versus control” group, we excluded the studies of Lawrence et al.28 and Fu et al.25 from the 10 selected articles because there were no “depression versus control” designs in these two studies. The selected 8 papers consisted of 238 subjects, 14 experiments, 50 conditions, and 132 foci of activations. The demographic data and characteristics of “depression versus control” selected articles are shown in Table 1. The demographic data and characteristics of “control versus depression” selected articles are shown in Table 2.
| Study | Subjects | Diagnostic Criteria | Patient Characteristics | Design (experimental task and condition) | Number of Foci | Statistical Threshold | Original Stereotaxic Space |
|---|---|---|---|---|---|---|---|
| Gotlib et al.21 | 18 patients versus 18 controls | DSM-IV | Depressed, medicated, recurrent | Sad versus neutral faces contrast; sex identification task (emotional [happy, sad, angry, fearful], neutral, and scrambled faces) | 1 | Uncorrected p <0.001 | Talairach and Tournoux |
| Keedwell et al.27 | 12 patients versus 12 controls | ICD–10 | Depressed, medicated, recurrent | Sad faces versus neural faces; mood-provocation paradigm; depression versus control contrast (all displaying mood-congruent [happy, sad, or neutral] facial expressions) | 7 | Uncorrected p=0.005 | Talairach and Tournoux |
| Surguladze et al.5 | 16 patients versus 14 controls | DSM-IV | Depressed, medicated, recurrent | Sad faces versus neutral faces; gender decision task; depression versus control contrast (20 happy, 20 neutral, and 20 sad faces) | 3 | Uncorrected p=0.003 | Talairach and Tournoux |
| Fu et al.4 | 19 patients versus 19 controls | DSM-IV | Depressed; drug-free, recurrent | Sad faces versus cross-hair fixation point; sad affect recognition task; depression versus control contrast (4 separate sessions: low, medium, high intensity of sad faces) | 89 | Uncorrected p <0.005 | Talairach and Tournoux |
| Chen et al.23 | 17 patients | DSM-IV | Depressed; medicated; recurrent | Sad faces versus cross-hair fixation points; Ekman series; Facial Affect-Processing Experiment; depression versus control contrast | 10 | Uncorrected p <0.005 | Talairach and Tournoux |
| Fu et al.24 | 19 patients and 19 controls | DSM-IV | Depressed, drug-naïve, first-episode | High-intensity sad faces versus cross-hair fixation-point; implicit sad facial-recognition task; depression versus control contrast (low, medium, high intensity of sad faces) | 13 | Uncorrected p<0.003 | Talairach and Tournoux |
| Fu et al.26 | 16 patients versus 16 controls | DSM-IV | Depressed, drug-free, recurrent | Sad faces versus cross-hair fixation-point; implicit sad facial-recognition task; depression versus control contrast (low, medium, high intensity of sad faces) | 9 | Uncorrected p<0.005 | Talairach and Tournoux |
| Suslow et al.29 | 30 patients, 26 controls | DSM-IV | Depressed; medicated, recurrent | Sad faces versus neutral faces; Ekman series; Facial Affect-Processing Experiment; depression versus control contrast | 1 | Uncorrected p=0.00039 | Montreal Neurological Institute |
| Study | Subjects | Diagnostic Criteria | Patient Characteristics | Design | Number of Foci | Statistical Threshold | Original Stereotaxic Space |
|---|---|---|---|---|---|---|---|
| Gotlib et al.21 | 18 patients versus 18 controls | DSM-IV | Depressed, medicated, recurrent | Sad-versus-neutral faces contrast; sex identification task; emotional (happy, sad, angry, fearful), neutral, and scrambled faces | 2 | Uncorrected p <0.001 | Talairach and Tournoux |
| Keedwell et al.27 | 12 patients versus 12 controls | ICD–10 | Depressed, medicated, recurrent | Sad faces versus neutral faces; mood-provocation paradigm; control versus depression contrast (all displaying mood-congruent {happy, sad, or neutral] facial expressions) | 3 | Uncorrected p=0.005 | Talairach and Tournoux |
| Lawrence et al.28 | 9 patients versus 11 controls | DSM-IV | Depressed; medicated; recurrent | Sad/neutral faces; facial identities task; control>depression contrast; (either sadness, happiness, or fear with different intensities) | 4 | Uncorrected p <0.005 | Talairach and Tournoux |
| Surguladze et al.5 | 16 patients versus 14 controls | DSM-IV | Depressed, medicated, recurrent | Sad faces; control versus depression contrast; gender decision task; 20 happy, 20 neutral, and 20 sad faces | 3 | Uncorrected p=0.003 | Talairach and Tournoux |
| Fu et al.4 | 19 patients versus 19 controls | DSM-IV | Depressed; drug-free, recurrent | Sad faces versus cross-hair fixation-point; sad affect recognition task; depression versus control contrast (4 separate sessions: low, medium, high intensity of sad faces) | 0 | Uncorrected p <0.005 | Talairach and Tournoux |
| Fu et al.25 | 19 patients versus 19 controls | DSM-IV | Depressed; medicated; recurrent | Sad faces versus cross-hair fixation-points; facial sad faces task; control versus depression contrast (low, medium, high intensity of emotional faces) | 19 | Uncorrected p <0.005 | Talairach and Tournoux |
| Fu et al.24 | 19 patients and 19 controls | DSM-IV | Depressed, drug-naïve, first-episode | High-intensity sad faces versus cross-hair fixation-points; sad facial recognition task; depression versus control contrast (low, medium, high intensity of sad faces) | 12 | Uncorrected p <0.005 | Talairach and Tournoux |
| Fu et al.26 | 16 patients versus 16 controls | DSM-IV | Depressed, drug-free, recurrent | Sad faces versus cross-hair fixation-points; implicit sad facial recognition task; depression versus control contrast (low, medium, high intensity of sad faces) | 17 | Uncorrected p <0.005 | Talairach and Tournoux |
In the “control versus depression” group, we excluded the studies of Chen et al.23 and Suslow et al.29 from the 10 articles because of the lack of “control versus depression” design in these articles. The 8 selected articles included 291 subjects, 18 experiments, 95 conditions, and 60 loci of activation.
Meta-analysis Procedure
We used the ALE theory as the background of our coordinate-based meta-analysis, which recognized the activation foci not as points but as spatial probability distributions centered at the given coordinates.30 The algorithm was modified to minimize within-experiment and within-group effects.31 Coordinates of foci, which were reported in the Montreal Neurological Institute (MNI) space, were converted to the Talairach space by the icbm2tal transformation algorithm32 before entering the following meta-analysis, which was performed with GingerALE software, Version 2.0 (Research Imaging Center; UT Health Science Center, San Antonio, TX), with better specificity and reasonable sensitivity.33,34 The ALE meta-analysis procedure consisted of four major steps. First, the software computed activation maps of each enrolled study. The model of Gaussian distributions was applied on all the foci of each study, and all these foci were merged into a single, three-dimensional volume. GingerALE software 2.0 used an uncertainty-modeling algorithm, rather than a pre-specified, full-width, half-maximum, smoothing kernel to empirically estimate the inter-subject and inter-exploratory variability in the neuroimaging experiments. Second, the individual modeled activation maps were united to compute the ALE values on a voxel-to-voxel basis, which was constrained to a gray-matter mask that defined the outer limit of the Talairach space. Third, an iterative permutation procedure (1011) was used by sampling each ALE result at an independently-chosen, random location, which would assess the above-chance clustering between experiments with null distribution of random spatial association to distinguish between noise and true convergences. This algorithm used a random-effects model to calculate the above-chance clustering between experiments, and then a fixed-effects model to calculate the above-chance clustering between foci. The iterative permutation was corrected for multiple comparison bias, using the false discovery rate method, with false-positive error p value <0.05. Fourth, a cluster analysis of thresholded maps was performed with the cluster-extent threshold of 200 mm3 criteria. Anatomical labels of these clusters were provided by the Talairach Daemon.35 Final ALE results were exported as NIFTI files into Mango Software (Research Imaging Center; UT Health Science Center, San Antonio, TX) and were overlaid onto an anatomical template generated by spatially normalizing the International Consortium for Brain Mapping template to Talairach space.
Results
Clusters of Activation in “Depression Versus Control” and “Control Versus Depression” Meta-Analysis
The “depression versus control” group showed significantly increased activations of likelihood in left striatum (volume: 1,920 mm3, Talairach coordinates [−19, 12, 7], ALE value: 2.54 × 10−3) and left PHG (volume: 1,472 mm3, Talairach coordinates [−11, −8, −10], ALE value: 3.02 × 10−3; Figure 1); the “control versus depression” group showed left MeFG (volume: 712 mm3, Talairach coordinates [−10, 24, 45], ALE value: 1.89 × 10−3); left MFG ([middle frontal gyrus] volume: 560 mm3, Talairach coordinates [−22, −28, 43]; ALE value: 1.88 × 10−3; right thalamus [volume: 816 mm3; Talairach coordinates [10, −21, 5]; ALE value: 1.71 × 10−3; left ACC [volume: 440 mm3, Talairach coordinates [0, 33, 16]; ALE value: [1.56 × 10−3] and SFG [volume: 408 mm3, Talairach coordinates [2, 30, 48]; ALE value: 1.34 × 10−3; [Figure 1]). All the significant clusters and statistical significance level reached a false discovery rate <0.05 and a cluster-extent threshold >200 mm3.
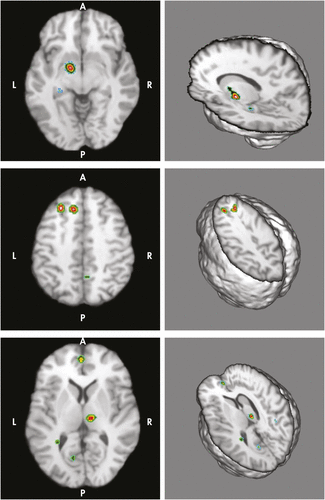
Upper panel showed significantly increased activations of likelihood in left striatum (Talairach coordinates [−19, 12, 7]) and left parahippocampal gyrus (Talairach coordinates [−11, −8, −10]) in the “Depression Versus Control” group. Middle and lower panels demonstrated the significant clusters of activations in “Control Versus Depression” group. Middle panel showed activations over left superior frontal gyrus (Talairach coordinates [2, 30, 48]) and left middle frontal gyrus (Talairach coordinates [−22, −28, 43]). Lower panel showed activations in left medial frontal gyrus (Talairach coordinates [−10, 24, 45]), right thalamus (Talairach coordinates [10, −21, 5]), left anterior cingulate cortex (Talairach coordinates [0, 33, 16]). Areas with hot (lighter) colors showed more statistically significant difference levels than those with cooler (darker) colors.
Discussion
From the above meta-analytic results, depression subjects recruit more neuronal activities in limbic regions, such as striatum and PHG. In contrast, controls have more activation of the frontal system (MFG, SFG, MeFG, ACC) and one limbic region (thalamus) during visual processing of emotions. Our findings in controls replicated the meta-analytic results of Steele et al., which showed MeFG activations while experiencing facial emotions.12 Our findings in depression patients also replicated the findings of Fitzgerald et al.’s meta-analysis, which showed decreased activities of the frontal system (ACC, MeFG) and increased activities of subcortical system (basal ganglion and hippocampus).2 Delaveau et al. also found frontal and subcortical dysregulations in their meta-analysis of emotion regulation in depression.6 Our findings correspond to the fronto-limbic hypothesis of depression.36 Besides, these regions also replicate the Sheline’s neuroanatomical concept of the limbic–cortical–striatal–pallidal–thalamic circuit, which is extensively interconnected.37 Striatum is usually correlated with emotional regulation and reward feedback.38,39 The increased striatal activities in depressed subjects during emotional recognition might represent the abnormalities of the “striatum” part of the fronto–striatal circuit.10,40,41 The abnormal activities probably represent the dysregulations in the identifications of emotions, compensation for lower motivation, or reward for social cognition and pathophysiology in the basal ganglion in empathetic experience of emotions.42,43 This concept is also supported by several studies showing that striatum dysfunction or damage would disturb the ability to recognize emotion in faces.44,45
Functions of the PHG include the conscious recollection and identification of facial features or emotions.46,47 The PHG also interacts with the amygdala to form the “emotion–memory” circuit and makes emotional memory more salient through enhancement of the encoding phase.9,48,49 Depressed patients have elevated PHG activities toward emotionally-relevant stimuli, but phase of depression and antidepressant treatment can attenuate the heightened responses in this area.50,51 Among the selected studies in this meta-analysis, Surguladze et al.’s reports revealed the increased PHG activities in depressed patients and linear increases of PHG response with exposure to sad faces.5 These studies support the PHG’s role in the identification of emotional facial features and possible enhanced retrieval or encoding of emotion-related memory when patients with depression perceived emotional faces.
The absence of amygdala activations in “depression versus controls” comparison might be explained because patients and controls might utilize the amygdala to a similar degree to produce the emotion-related responses. Besides, Grant et al.52 thought that amygdalar activations might be more significant in depression comorbid with childhood trauma, not pure depression. Finally, the low number of foci in amygdalae in these selected articles might contribute to the findings of absent amygdalar activations.
The control subjects showed more utilization of the frontal system and thalamus than depressed patients, which might suggest different mechanisms between the two groups in managing facial expression of emotions. The frontal executive system is impaired in the depressed patients, and executive dysfunction usually interrelates cognition with mood in visual processing of emotional faces.53 Buchsbaum et al. mentioned that decreased metabolisms over MFG, MeFG, ACC, SFG, and thalamus of depressed patients could be reversed by antidepressant treatment,54,55 which might suggest that these areas are state-markers for depression; our findings also replicated these results. MFG, MeFG, SFG, and ACC are associated with visual/emotional integration, and these areas interact with each other to establish the central network for emotional face recognition, empathic face-to-face interaction, and emotional interpersonal cognition.56–59 Kilts et al. found that MFG, MeFG, and SFG might be responsible for the judgments and recognitions of facial emotions.60 Naismith et al. also reported that controls would activate MFG more than depressed patients in an implicit-learning task,61 which might be related to implicit emotional meaning of our meta-analysis. SFG might also contribute to visual attention deficits and emotional context encoding while focusing on the interpretations for the emotions.62–65 ACC is important for emotional and cognitive conflict resolution,66,67 rapid processing of salient facial emotional information or response to emotional faces,11,16,21,68,69 emotional self- regulation capacity,43,70,71 visceral emotional responses to aversive visual stimuli,18 visual attention,72 and empathic or associative learning of emotions.13 Controls might have superior ability to activate the ACC for regulating visual processing of emotional faces and conflict resolution when seeing emotional faces.
The thalamus is one part of limbic–cortico–striato–pallidal–thalamic circuit in depression pathogenesis. However, higher activities of the thalamus were observed in this meta-analysis. This can be explained in that the frontal system might work with the thalamus to control other limbic regions (i.e., striatum and PHG) for the responses of facial emotion. This “top-down” emotional network could be the pathophysiological model for our meta-analytic results.73 The thalamus probably interacts with striatum or PHG through thalamo-striatal and thalamo-limbic circuits to facilitate the control over limbic primitive responses for emotional faces.74,75
There are several limitations in this meta-analytic study. First, the age, medication dose and type, actively depressed or remitted status, gender, acquisition MR strengths, and intensity scores of activations in the clusters were not controlled by covariate analysis of these enrolled studies. For example, medication (i.e., antidepressant) effects in the brain is an important issue for possible biases in such a study. Antidepressants might influence cortico–subcortical connectivity and functional activities in healthy volunteers.76 The existence of medication might limit us to confirm the findings here likely to be related to depression, and not secondary to medication. Psychopathological status is another important confounder because actively-depressed and remitted-depressed patients might have different expressions of brain function or structure.77 These should be considered as covariates in the further ALE meta-analysis. Second, the number of enrolled studies seems relatively lower, which might limit the power of results.78 Also, the foci in our meta-analysis would be inadequate (132 foci and 60 foci, respectively) for the typical meta-analysis. Our meta-analytic results might suffer from limited power because of the limited number of coordinates and enrolled studies. Third, baseline tasks to which the sad faces were compared in these selected studies are different (i.e., neutral faces, cross-hair fixation-point, or scrambled faces). This can have a major effect on activation, particularly in limbic areas,79 which might be related to the absence of significant amygdala findings in the current meta-analysis.
Conclusions
From the meta-analytic results, we could conclude that depressive patients might have higher activation in limbic regions, and controls might have higher fronto–thalamic activations during visual processing of emotional faces.
1 : Explicit and incidental facial expression processing: an fMRI study. Neuroimage 2001; 14:465–473Crossref, Medline, Google Scholar
2 : A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp 2008; 29:683–695Crossref, Medline, Google Scholar
3 : Frontolimbic responses to emotional face memory: the neural correlates of first impressions. Hum Brain Mapp 2009; 30:3748–3758Crossref, Medline, Google Scholar
4 : Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry 2004; 61:877–889Crossref, Medline, Google Scholar
5 : A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 2005; 57:201–209Crossref, Medline, Google Scholar
6 : Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J Affect Disord 2011; 130:66–74Crossref, Medline, Google Scholar
7 : Automatic brain response to facial emotion as a function of implicitly and explicitly measured extraversion. Neuroscience 2010; 167:111–123Crossref, Medline, Google Scholar
8 : Expecting unpleasant stimuli: an fMRI study. Psychiatry Res 2007; 154:1–12Crossref, Medline, Google Scholar
9 : Emotions in motion: dynamic compared to static facial expressions of disgust and happiness reveal more widespread emotion-specific activations. Brain Res 2009; 1284:100–115Crossref, Medline, Google Scholar
10 : Frontostriatal deficits in unipolar major depression. Brain Res Bull 1998; 47:297–310Crossref, Medline, Google Scholar
11 : Specific brain processing of facial expressions in people with alexithymia: an H2 15O-PET study. Brain 2003; 126:1474–1484Crossref, Medline, Google Scholar
12 : Prefrontal cortical functional abnormality in major depressive disorder: a stereotactic meta-analysis. J Affect Disord 2007; 101:1–11Crossref, Medline, Google Scholar
13 : Differential effects of tryptophan depletion on emotion processing according to face direction. Soc Cogn Affect Neurosci 2007; 2:264–273Crossref, Medline, Google Scholar
14 : Functional MRI changes before and after onset of reported emotions. Psychiatry Res 2004; 132:239–250Crossref, Medline, Google Scholar
15 : Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain 2003; 126:1691–1712Crossref, Medline, Google Scholar
16 : Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. J Child Psychol Psychiatry 2003; 44:1015–1024Crossref, Medline, Google Scholar
17 : Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc Cogn Affect Neurosci 2009; 4:387–398Crossref, Medline, Google Scholar
18 : Reduced left subgenual anterior cingulate cortical activity during withdrawal-related emotions in melancholic depressed female patients. J Affect Disord 2010; 127:326–331Crossref, Medline, Google Scholar
19 : The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 2003; 160:64–75Crossref, Medline, Google Scholar
20 : Affective context interferes with cognitive control in unipolar depression: an fMRI investigation. J Affect Disord 2009; 114:131–142Crossref, Medline, Google Scholar
21 : Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport 2005; 16:1731–1734Crossref, Medline, Google Scholar
22 : Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012Crossref, Medline, Google Scholar
23 : Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry 2007; 62:407–414Crossref, Medline, Google Scholar
24 : Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biol Psychiatry 2008; 63:656–662Crossref, Medline, Google Scholar
25 : Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry 2007; 164:599–607Crossref, Medline, Google Scholar
26 : Neural responses to sad facial expressions in major depression following cognitive-behavioral therapy. Biol Psychiatry 2008; 64:505–512Crossref, Medline, Google Scholar
27 : A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry 2005; 58:495–503Crossref, Medline, Google Scholar
28 : Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 2004; 55:578–587Crossref, Medline, Google Scholar
29 : Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry 2010; 67:155–160Crossref, Medline, Google Scholar
30 : Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 2002; 16:765–780Crossref, Medline, Google Scholar
31 : Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp 2012;331–13Crossref, Medline, Google Scholar
32 : Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 2007; 28:1194–1205Crossref, Medline, Google Scholar
33 : Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009; 30:2907–2926Crossref, Medline, Google Scholar
34 : ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 2005; 25:155–164Crossref, Medline, Google Scholar
35 : Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000; 10:120–131Crossref, Medline, Google Scholar
36 : Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry 2008; 63:569–576Crossref, Medline, Google Scholar
37 : 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry 2000; 48:791–800Crossref, Medline, Google Scholar
38 : Reward, addiction, and emotion regulation systems associated with rejection in love. J Neurophysiol 2010; 104:51–60Crossref, Medline, Google Scholar
39 : Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry 1995; 152:341–351Crossref, Medline, Google Scholar
40 : Impaired implicit sequence learning in depression: a probe for frontostriatal dysfunction? Psychol Med 2006; 36:313–323Crossref, Medline, Google Scholar
41 : Orbitofrontal volume reductions during emotion recognition in patients with major depression. J Psychiatry Neurosci 2010; 35:311–320Crossref, Medline, Google Scholar
42 : Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry 2006; 163:1784–1790Link, Google Scholar
43 : Neurobiology of emotion perception, II: implications for major psychiatric disorders. Biol Psychiatry 2003; 54:515–528Crossref, Medline, Google Scholar
44 : Impaired recognition of anger following damage to the ventral striatum. Brain 2004; 127:1958–1969Crossref, Medline, Google Scholar
45 : Specific impairments in the recognition of emotional facial expressions in Parkinson’s disease. Neuropsychologia 2008; 46:2300–2309Crossref, Medline, Google Scholar
46 : Dissociable neural responses in the hippocampus to the retrieval of facial identity and emotion: an event-related fMRI study. Hippocampus 2003; 13:429–436Crossref, Medline, Google Scholar
47 : Children recruit distinct neural systems for implicit emotional face processing. Neuroreport 2006; 17:215–219Crossref, Medline, Google Scholar
48 : The locus coeruleus is involved in the successful retrieval of emotional memories in humans. J Neurosci 2006; 26:7416–7423Crossref, Medline, Google Scholar
49 : “Change the mind and you change the brain”: effects of cognitive-behavioral therapy on the neural correlates of spider phobia. Neuroimage 2003; 18:401–409Crossref, Medline, Google Scholar
50 : Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: a randomized cross-over study. Neuroimage 2010; 49:1161–1170Crossref, Medline, Google Scholar
51 : Effect of bupropion extended release on negative emotion-processing in major depressive disorder: a pilot functional magnetic resonance imaging study. J Clin Psychiatry 2007; 68:261–267Crossref, Medline, Google Scholar
52 : Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatr Res 2011; 45:886–895Crossref, Medline, Google Scholar
53 : Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res 2008; 163:143–155Crossref, Medline, Google Scholar
54 : Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry 1997; 41:15–22Crossref, Medline, Google Scholar
55 : Changes in regional brain activity in major depression after successful treatment with antidepressant drugs. Acta Psychiatr Scand 1998; 98:54–59Crossref, Medline, Google Scholar
56 : Processing of disgusted faces is facilitated by odor primes: a functional MRI study. Neuroimage 2010; 53:746–756Crossref, Medline, Google Scholar
57 : Word wins over face: emotional Stroop effect activates the frontal cortical network. Front Hum Neurosci 2011; 4:234Crossref, Medline, Google Scholar
58 : Emotional perception: meta-analyses of face and natural scene processing. Neuroimage 2011; 54:2524–2533Crossref, Medline, Google Scholar
59 : Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J Cogn Neurosci 2007; 19:1354–1372Crossref, Medline, Google Scholar
60 : Dissociable neural pathways are involved in the recognition of emotion in static and dynamic facial expressions. Neuroimage 2003; 18:156–168Crossref, Medline, Google Scholar
61 : Fronto-striatal correlates of impaired implicit sequence learning in major depression: an fMRI study. J Affect Disord 2010; 125:256–261Crossref, Medline, Google Scholar
62 : Neural correlates of attention biases of people with major depressive disorder: a voxel-based morphometric study. Psychol Med 2009; 39:1097–1106Crossref, Medline, Google Scholar
63 : Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage 2010; 50:347–356Crossref, Medline, Google Scholar
64 : Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry 2005; 62:397–408Crossref, Medline, Google Scholar
65 : Incidental retrieval of emotional contexts in post-traumatic stress disorder and depression: an fMRI study. Brain Cogn 2009; 69:98–107Crossref, Medline, Google Scholar
66 : Interference produced by emotional conflict associated with anterior cingulate activation. Cogn Affect Behav Neurosci 2006; 6:152–156Crossref, Medline, Google Scholar
67 : Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. J Psychiatry Neurosci 2008; 33:199–208Medline, Google Scholar
68 : Involvement of the anterior cingulate and frontoinsular cortices in rapid processing of salient facial emotional information. Neuroimage 2011; 54:2539–2546Crossref, Medline, Google Scholar
69 : Mood alters amygdala activation to sad distractors during an attentional task. Biol Psychiatry 2006; 60:1139–1146Crossref, Medline, Google Scholar
70 : The anterior cingulate cortex: the evolution of an interface between emotion and cognition. Ann N Y Acad Sci 2001; 935:107–117Crossref, Medline, Google Scholar
71 : Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport 2006; 17:843–846Crossref, Medline, Google Scholar
72 : Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 2002; 26:321–352Crossref, Medline, Google Scholar
73 : An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia 2003; 41:585–596Crossref, Medline, Google Scholar
74 : Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron 2010; 67:294–307Crossref, Medline, Google Scholar
75 : Antipsychotics increase vesicular glutamate transporter 2 (VGLUT2) expression in thalamo-limbic pathways. Neuropharmacology 2008; 54:497–508Crossref, Medline, Google Scholar
76 : Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. Neuroimage 2011; 57:1317–1323Crossref, Medline, Google Scholar
77 : A subtle grey-matter increase in first-episode, drug-naive major depressive disorder with panic disorder after 6 weeks’ duloxetine therapy. Int J Neuropsychopharmacol 2011; 14:225–235Crossref, Medline, Google Scholar
78 : Pooling FMRI data: meta-analysis, mega-analysis and multi-center studies. Front Neuroinform 2009; 3:33Crossref, Medline, Google Scholar
79 : Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Brain Res Rev 2008; 58:57–70Crossref, Medline, Google Scholar



