Neurobiology of Implicit and Explicit Bias: Implications for Clinicians
Stigma and bias associated with mental illnesses present challenges to both patients and clinicians. The recent emergence of the interdisciplinary field of social cognitive neuroscience enables exploration of cultural, neuroanatomical, and cognitive processes that underlie activation and regulation of stigma and bias. This field combines techniques from multiple disciplines including functional imaging and social observation to provide a richer understanding of social behavior and perceptions. These, in turn, have the potential to inform development and implementation of more effective approaches to reducing the negative effects of stigma.
Stigma can be separated into two types: external and internal.13–15 External (public, societal) stigma is conceptualized as a negative belief or attitude (bias) that the general population holds about individuals identified as members of the stigmatized group. Internal (self) stigma is defined as the construct that occurs when the external stigma is also believed (internalized) by a member of the stigmatized group. Explicit attitudes and biases are within an individual’s conscious awareness. An individual can reflect on and monitor these easily. Implicit attitudes and biases are the automatically activated unconscious counterparts of self-reported explicit attitudes. In some situations, implicit attitudes predict behaviors better than explicitly held beliefs.16,17
There is increasing evidence that both implicit (unconscious) and explicit (conscious) biases can have detrimental effects on mental health care outcomes.14 Activation of negative beliefs about a group (stigma) can cause many types of negative behaviors, including overt discrimination and a desire for increased social distance. Stigmas commonly associated with mental illness include that such individuals are “bad” (likely to act in dangerous, violent, or unpredictable ways), “blameworthy” (responsible for their illness, morally weak), and “helpless” (incompetent to achieve life goals).14,18 A recent study reported that mental illness had an even stronger implicit association with disease than danger, suggesting another possible basis for distancing/avoidance behaviors.18 It is well established in the literature that internalization of societal stigma (self-stigma) is associated with multiple detrimental effects, including lower self-esteem, higher sense of hopelessness, and greater resistance to engage in treatment.14,19,20 Thus, self-stigma associated with mental illness creates resistance to admitting that a psychological problem exists, and negative beliefs about mental health care create resistance to entering or continuing treatment. These constructs interact with other barriers to care (e.g., not knowing where to get help, inadequate transportation, scheduling difficulties, difficulty getting time off, cost) to decrease treatment utilization. Recent studies showed that self-stigma and fear of societal stigma increased the likelihood of treatment dropout and decreased the likelihood of treatment utilization in military populations.21,22
Although less well studied, clinician-held biases can compromise both a patient’s willingness to accept services and the quality of clinical care that patients receive.4,14,23–26 Aspects of clinician behavior that can discourage patients from seeking or continuing treatment include communication styles that are negative or condescending (patronizing, dismissive) and/or emphasis on limitations and barriers (pessimism about recovery) rather than strengths and successes.14,26 Studies in mental health clinicians have reported that implicit bias predicted overdiagnosis (pathologizing) and selection of more restrictive and controlling interventions.23,25 Explicit bias predicted a more negative prognosis.23
A fundamental aspect of social interaction and social cognition is the rapid identification of others as either similar to (in-group) or different from (out-group) the self.27–31 This type of social/affective decision making utilizes heuristics to automatically categorize individuals based on easily observable traits, factors, or characteristics (e.g., ethnicity, gender, age, weight, speech, attire, profession, hobbies, grooming). Use of heuristics is an essential aspect of very rapid decision making, as is required in most social interactions.31,32 Such categorizations are culturally based stereotypes that are learned associations (semantic memory) among specific groups of people and specific traits and attributes.13,29,33 Cultural stereotypes result in preconceptions (prejudices, biases) that influence expectations and/or evaluations in both positive (halo effect) and negative (stigma) ways.13,29,31,34 Studies have shown that an individual’s explicit (conscious) and implicit (unconscious) beliefs can be incongruent; implicit beliefs often predict behavioral expression better than do explicit beliefs.17
Cultural stereotypes provide a basis for inferring information about an individual based on a category. Such prejudices and biases can result in changes in behavior (discrimination), which can be positive or negative. Thus, individuals perceived as out-group are more likely to be seen as threatening or hostile (negative bias), whereas individuals perceived as in-group are more likely to be seen as trustworthy or friendly (positive bias).28,35 One prominent model of stereotypes utilizes two dimensions: warmth and competence (Figure 1).1–3,5 Across cultures, groups that an individual identifies with (in-group) generally are stereotyped as high on both warmth and competence, setting a strongly positive bias. Expectations include that members of the group are capable and well intentioned, which promotes trust and respect. By contrast, groups viewed as outcasts are stereotyped as low on both warmth and competence, setting a strongly negative bias. Expectations include that members of an outcast group are hostile and unreliable, which promotes contempt and disgust. When considered as a general category, people with mental illness were rated somewhat low on both warmth and competence, similar on both dimensions to people who were poor.5 When different disorders were considered, individuals suffering from anxiety or eating disorders were seen by study participants as relatively more competent and warm than individuals with serious mental illnesses such as schizophrenia or sociopathy.3,5 There is evidence that impression formation can be more strongly influenced by stereotypic expectations than knowledge about the individual.11 This stereotypic expectation can be directly related to the sometimes stigmatizing culture among clinicians treating mental illness.26 If a clinician approaches a patient with a perception that mental illness is a permanent disability, stereotypic expectation of incompetence may be activated.1,3,4 By contrast, a recovery-oriented mindset is more likely to increase the expectation of achieving goals.
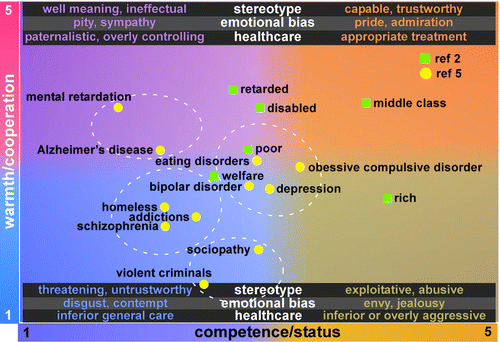
FIGURE 1. Cultural stereotypes are used to infer information about an individual based on how they are categorized. Prejudices and biases derived from the applied stereotype may result in predictable changes in behavior (i.e., discrimination). The stereotype content model systematizes stereotypes along the social judgment dimensions of warmth and competence.1–5 The dimension of warmth rates intentions (benevolent, malevolent) and provides a basis for inferring whether someone will be a friend or enemy. The dimension of competence rates the ability to enact intentions (capable, ineffectual) and correlates very highly with status across cultures. The two dimensions separate groups into four clusters, each associated with a particular combination of expectations and emotional biases. These, in turn, can influence behaviors, including delivery of clinical care.4 Groups stereotyped as high on warmth but low on competence, such as individuals with intellectual disabilities, may be institutionalized unnecessarily. Groups stereotyped as low on both dimensions, such as homeless individuals, are more likely to receive a lower level of care in general. A study assessing where groups with mental illnesses fell within this framework reported that when evaluated as a category, people with mental illnesses were rated very much like poor people, somewhat low on both warmth and competence.5 When specific mental illnesses were rated, the categories of anxiety or eating disorders were rated as relatively more competent and warm than the serious mental illnesses such as schizophrenia or sociopathy.5
There are many theories for how social decision making is accomplished in the brain. Both fast automatic processes (as described above) and higher-order reasoning processes that are slower and require cognitive effort can occur.36,37 Many dual-system theories of decision making have hypothesized that emotion and reason are supported by separate systems that are in competition (parallel-competitive, heart versus head, hot versus cold).36,37 An alternative theory (default-interventionist) is that fast autonomous processes (type 1; heuristic based, intuitive, little or no working memory required) provide default decisions unless higher-order reasoning processes (type 2; reflective, flexible, working memory dependent) intervene.36 A compatible conceptualization is that multiple interacting processes are more congruent with recent research than a dual-system conceptualization.37–40 In this view, the modulatory effects of emotion (implicit or explicit) on decision making and the importance of emotion (affect) in computation of subjective value are emphasized in addition to the regulation of emotion by cognitive processes.
The amygdala is consistently implicated in the fast automatic evaluation of socially relevant stimuli.28,29,31,37,41 This area is a key structure for rapidly identifying and adaptively responding to a wide range of behaviorally salient events. Although the amygdala is most commonly studied utilizing events with a negative valence (e.g., angry faces, fear-based learning), the amygdala is also responsive to positive events (e.g., happy faces, reward-based learning). Subliminal presentation (most commonly done by backward masking and/or extremely brief presentation) is one approach to assessing the neural correlates of automatic (unconscious) processing. A meta-analysis of functional magnetic resonance imaging (fMRI) studies reported that the only area consistently activated by subliminal presentation of emotional faces was the amygdala (bilateral).42 Studies utilizing extremely brief stimulus presentations to manipulate context immediately before task presentation have demonstrated priming-induced changes in behavioral and/or neural responses. Such biases are presumed to be mediated by automatically evoked processes. For example, a recent study reported that neutral stimuli were rated more likeable after subliminal presentation of positive affective primes and less likeable after subliminal presentation of negative affective primes.43 Similarly, when context was manipulated by presenting a race label before the presentation of face stimuli to be categorized, both categorization and event-related potentials were affected.44 A recent study reported that the amygdala was responsive to both subliminal and supraliminal presentations of faces that varied on trustworthiness, suggesting that modulation by complex social cues may occur even in the absence of awareness.45
Some studies of racial prejudice have reported that activation of the amygdala was greater when white participants viewed black faces compared with white faces, particularly if the presentation was very brief.29,31,46 The competing interpretations are that this is a result of fear-based learning or of threat detection.29,46 A study contrasting viewing emotional expressions (angry, fearful, happy) of in-group (own-culture) and out-group (other-culture) individuals reported that amygdala activation was comparatively stronger only when viewing fear expressions of own-culture individuals.47 As noted by the authors of this study, these results are consistent with higher vigilance toward own-group fear because it potentially signals proximal danger (threat detection). Manipulation of the task goal while viewing faces of the same race or other races also produced changes in amygdala activation that were goal relevant rather than race related.29,48 A meta-analysis of fMRI studies of social categorizations reported that the amygdala was activated by presentation of both in-group and out-group conditions.28 As noted in a recent review, studies utilizing the minimal groups paradigm (novel in-group/out-group designations based on arbitrary factors) have demonstrated that in-group/out-group determinations are highly dynamic and malleable.31 Of particular relevance, this approach provides a way to assess the neural correlates of in-group/out-group reactions without contamination from cultural stereotypes. Under minimal group conditions, amygdala activation was higher in response to in-group faces than out-group faces, regardless of race.31
Multiple lines of evidence suggest that social (person-specific) knowledge including social categorization (knowledge about social groups) is a unique type of semantic knowledge that is different from general knowledge about living things.6–8 Studies have reported that the areas activated by tasks requiring social categorization (features associated with types of people) are different from tasks requiring other types of categorization (features associated with types of objects or types of places) (Figure 2).8,9,11 Identifying features with the social category evoked greater activation in areas commonly associated with social cognition (medial prefrontal cortex, posterior cingulate/precuneus, temporoparietal junction, and anterior temporal cortex).8,9,11 By contrast, identifying features with the objects category evoked greater activation in areas commonly associated with general semantic processing (lateral inferior frontal and inferior temporal cortices).8 Identifying features with the places category evoked greater activation in areas commonly associated with spatial processing (parahippocampal gyrus and retrosplenial cortex).9 A meta-analysis of functional imaging studies addressing some aspect of reasoning about mental states (theory of mind, mentalizing, mind reading) reported that activations associated with tasks requiring use of conceptual knowledge about people (trait judgment) were most convergent on the same set of areas (Figure 2).10
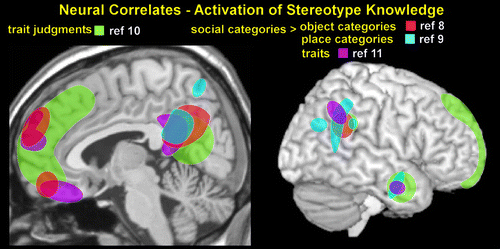
FIGURE 2. Functional magnetic resonance imaging and lesion-deficit studies indicate that person-specific knowledge including cultural stereotypes (categories of people) may be a unique type of semantic knowledge.6,7 The results of several studies assessing neural activations during tasks requiring use of stereotype knowledge are overlaid on lateral and medial magnetic resonance images.8–11 The areas most commonly activated by tasks requiring use of person-specific categorical knowledge are the medial prefrontal cortex, posterior cingulate cortex/precuneus, temporoparietal junction, and anterior temporal lobes.
Social categorization is complex because different attributes of an individual may trigger multiple stereotypes.8,28,29,31,49,50 In one context, the key factor might be race or gender; in another context, the key factor might be which sports team an individual supports, where the person resides, or what the individual does for a living. In addition, any incongruities between expectations based on the applied stereotype and appearance and/or actions of the individual being judged (counter-stereotypical traits, trait violations, trait incongruency) are likely to evoke reconsideration. Multiple studies support activation of the dorsal anterior cingulate cortex (ACC) in conflict detection and engagement of the dorsolateral prefrontal cortex (PFC) for executive (top-down) control in support of goal maintenance.29,39 Conflicting social information can engage the dorsal ACC without conscious awareness (implicit, automatic) of conflict.29,50,51 A recent study that also quantified each participant’s level of implicit bias reported that this correlated positively with the level of activation within the dorsolateral PFC during incongruent trials.50 As noted by the authors, these results are consistent with detection of a higher degree of conflict triggering greater recruitment of executive resources to override negative bias in order to respond correctly. Another study that varied emotional valence and attentional demands reported that incongruent trials were associated with greater connectivity between the dorsal ACC and both the amygdala and dorsolateral PFC, further supporting the involvement of these areas in the detection and regulation of implicit bias.51
The ACC and the dorsolateral PFC are also implicated in the voluntary regulation of negative bias.29 An fMRI study comparing the neural correlates of response regulation while viewing images of individuals from stigmatized groups (homeless people, alcoholics) and images with negative social content unrelated to stigma reported intriguing differences (Figure 3).12 No areas were more activated during the viewing of stigma-related images than non–stigma-related images for either instruction condition (maintain versus intentionally decrease negative emotional responses) when the entire 8 seconds of viewing was utilized. By contrast, the analysis that utilized only the first 2 seconds showed higher activations in multiple areas within the PFC (dorsolateral, ventrolateral, orbital, medial, ACC) when participants intentionally decreased negative affect while viewing stigma-related images compared with non–stigma-related images. In addition, measures of implicit bias correlated with activation level in several of these areas. As noted by the authors, these results are consistent with previous studies supporting engagement of the PFC during downregulation of negative bias, and also suggest that the time course of stigma responding may be faster than that of general emotion regulation.
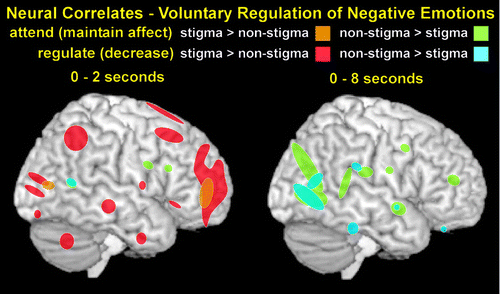
COVER and FIGURE 3. A functional magnetic resonance imaging study that assessed the neural correlates of response regulation during the viewing of images with negative social content reported differences by whether the images were of individuals from stigmatized groups (homeless persons, alcoholics).12 When the entire viewing period of 8 seconds was compared (image on right of panel), there were no areas more activated by stigma-related images than by non–stigma-related images. When only the initial 2 seconds was compared (image on left), multiple areas were activated more by stigma-related images in both task conditions (maintain negative affect, decrease negative affect) including several in the prefrontal cortex. In addition, measures of implicit bias correlated with activation level in several of these areas (cover). These results support automatic engagement of executive control during the viewing of stigma-related images, because the lateral prefrontal cortex was activated in both task conditions. These results also suggest that the time course of stigma responding may be faster than that of general emotion regulation.
Explicit (conscious) aspects of social context have been shown to modulate both emotional and neural responses to stigmatized individuals. Belief that an individual experiencing pain bears personal responsibility for having AIDS decreases both empathy ratings and neural activations in areas associated with empathy (anterior insula, dorsal ACC, periaqueductal gray).52,53 Similarly, a study reported differences in neural activations associated with viewing stigmatized individuals based on whether they were considered to be responsible for being homeless.54 After controlling for activations related to reading the scenarios, viewing blamed individuals was associated with higher activations in the PFC areas implicated in valuation (orbital) and social cognition (medial). By contrast, viewing blameless individuals was associated with higher activations in the PFC areas implicated in cognitive control (dorsolateral, ventrolateral) and empathy (insula). These results support blame reduction as a useful target for interventions to reduce stigma and prejudice related to mental illness.
Automatic assessments of external stimuli are essential for survival in a rapidly changing social environment.27–31 Therefore, one approach to reducing stigma is to change the social stereotype. Internationally, there have been multiple efforts to educate the public about the neurobiological bases of mental illnesses, with the intention of diminishing stigma by equating mental and physical illnesses.55 Overall, studies do not support this as an effective approach. Although educating the public about neurobiological models of mental illness may decrease blaming, it can increase other negative attitudes and beliefs.55–57 This can be true even for mental health clinicians, as indicated by a recent study reporting that empathy ratings for patient vignettes were lower when symptoms were attributed to biological compared with psychosocial causes.57 Interventions directed toward modifying habitual responding may be more successful.13,24,58 The two primary approaches to reducing expression of stigma and prejudice at the individual level are to reduce the activation of implicit stereotyping (decrease automatic responding) and to change perception of others by purposefully restructuring thoughts (increase cognitive control).13 A major approach to decreasing activation of implicit stereotyping is to increase awareness of information that is specific to the individual (individuation), particularly any that counters some aspect of stereotype.24,58 It has been shown that negative bias is greater in people with little exposure to mental health care and/or individuals suffering from mental illness (reliance on stereotype), supporting the value of personal experiences in disconfirming stereotypes.23,26,58 Clinicians can defend against the possible influence of stereotypes and bias by practicing purposeful individuation when treating patients, focusing on the individual rather than the category. Use of person-centered language when speaking with patients will also increase individuation. Motivational interviewing strategies may also enhance perception of the patient as competent, thus countering the incompetence stereotype.3 In addition, motivational interviewing and using person-centered language provide the patients with an opportunity to see themselves as more competent and active members of their treatment team, which may decrease self-stigma and increase treatment compliance and utilization. Clinicians have an opportunity to use many different techniques to combat implicit and explicit bias, thus reducing prejudice and creating a recovery-oriented environment for patient care.
1 : A model of (often mixed) stereotype content: competence and warmth respectively follow from perceived status and competition. J Pers Soc Psychol 2002; 82:878–902Crossref, Medline, Google Scholar
2 : Universal dimensions of social cognition: warmth and competence. Trends Cogn Sci 2007; 11:77–83Crossref, Medline, Google Scholar
3 : Warmth and competence: stereotype content issues for clinicians and researchers. Can Psychol 2012; 53:14–20Crossref, Medline, Google Scholar
4 : Under the radar: how unexamined biases in decision-making processes in clinical interactions can contribute to health care disparities. Am J Public Health 2012; 102:945–952Crossref, Medline, Google Scholar
5 : Stereotypes of mental disorders differ in competence and warmth. Soc Sci Med 2012; 74:915–922Crossref, Medline, Google Scholar
6 : Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci 2013; 8:123–133Crossref, Medline, Google Scholar
7 : The neural network associated with lexical-semantic knowledge about social groups. Cortex 2015; 70:155–168Crossref, Medline, Google Scholar
8 : Dissociable neural correlates of stereotypes and other forms of semantic knowledge. Soc Cogn Affect Neurosci 2012; 7:764–770Crossref, Medline, Google Scholar
9 : Category-selective neural substrates for person- and place-related concepts. Cortex 2013; 49:2748–2757Crossref, Medline, Google Scholar
10 : Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev 2014; 42:9–34Crossref, Medline, Google Scholar
11 : Distinct neural correlates of social categories and personality traits. Neuroimage 2015; 104:336–346Crossref, Medline, Google Scholar
12 : How does the brain regulate negative bias to stigma? Soc Cogn Affect Neurosci 2012; 7:715–726Crossref, Medline, Google Scholar
13 : Stereotypes, prejudice, and depression: the integrated perspective. Perspect Psychol Sci 2012; 7:427–449Crossref, Medline, Google Scholar
14 : The impact of mental illness stigma on seeking and participating in mental health care. Psychol Sci Public Interest 2014; 15:37–70Crossref, Medline, Google Scholar
15 : Self-stigma regarding mental illness: definition, impact, and relationship to societal stigma. Psychiatr Rehabil J 2015; 38:99–102Crossref, Medline, Google Scholar
16 : The neural basis of implicit attitudes. Curr Dir Psychol Sci 2008; 17:164–170Crossref, Google Scholar
17 : Implicit social cognition: from measures to mechanisms. Trends Cogn Sci 2011; 15:152–159Crossref, Medline, Google Scholar
18 : Sick in the head? Pathogen concerns bias implicit perceptions of mental illness. Evol Psychol 2014; 12:706–718Crossref, Medline, Google Scholar
19 : Implicit self-stigma in people with mental illness. J Nerv Ment Dis 2010; 198:150–153Crossref, Medline, Google Scholar
20 : Stigma as a barrier to recovery from mental illness. Trends Cogn Sci 2012; 16:9–10Crossref, Medline, Google Scholar
21 : The role of different stigma perceptions in treatment seeking and dropout among active duty military personnel. Psychiatr Rehabil J 2015; 38:142–149Crossref, Medline, Google Scholar
22 : Barriers and facilitators of mental health treatment-seeking in U.S. active duty soldiers with sexual assault histories. J Trauma Stress 2015; 28:289–297Crossref, Medline, Google Scholar
23 : Implicit and explicit stigma of mental illness: links to clinical care. J Nerv Ment Dis 2008; 196:752–760Crossref, Medline, Google Scholar
24 : Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med 2013; 28:1504–1510Crossref, Medline, Google Scholar
25 : Implicit and explicit stigma of mental illness: attitudes in an evidence-based practice. J Nerv Ment Dis 2013; 201:1072–1079Crossref, Medline, Google Scholar
26 : Prejudice and discrimination from mental health service providers. Psychiatr Rehabil J 2015; 38:203–206Crossref, Medline, Google Scholar
27 : Attitudes and evaluations: a social cognitive neuroscience perspective. Trends Cogn Sci 2007; 11:97–104Crossref, Medline, Google Scholar
28 : Is social categorization based on relational ingroup/outgroup opposition? A meta-analysis. Soc Cogn Affect Neurosci 2013; 8:870–877Crossref, Medline, Google Scholar
29 : The neuroscience of prejudice and stereotyping. Nat Rev Neurosci 2014; 15:670–682Crossref, Medline, Google Scholar
30 : The rules of implicit evaluation by race, religion, and age. Psychol Sci 2014; 25:1804–1815Crossref, Medline, Google Scholar
31 : The neuroscience of intergroup relations: an integrative review. Perspect Psychol Sci 2014; 9:245–274Crossref, Medline, Google Scholar
32 : Reexamining our bias against heuristics. Adv Health Sci Educ Theory Pract 2014; 19:457–464Crossref, Medline, Google Scholar
33 : The spontaneous formation of stereotypes via cumulative cultural evolution. Psychol Sci 2014; 25:1777–1786Crossref, Medline, Google Scholar
34 : With malice toward none and charity for some: ingroup favoritism enables discrimination. Am Psychol 2014; 69:669–684Crossref, Medline, Google Scholar
35 : Self-protective biases in group categorization: threat cues shape the psychological boundary between “us” and “them”. J Pers Soc Psychol 2010; 99:62–77Crossref, Medline, Google Scholar
36 : Dual-process theories of higher cognition: advancing the debate. Perspect Psychol Sci 2013; 8:223–241Crossref, Medline, Google Scholar
37 : Emotion and decision making: multiple modulatory neural circuits. Annu Rev Neurosci 2014; 37:263–287Crossref, Medline, Google Scholar
38 : Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci Biobehav Rev 2012; 36:479–501Crossref, Medline, Google Scholar
39 : Emotional foundations of cognitive control. Trends Cogn Sci 2015; 19:126–132Crossref, Medline, Google Scholar
40 : Follow the heart or the head? The interactive influence model of emotion and cognition. Front Psychol 2015; 6:573Crossref, Medline, Google Scholar
41 : Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 2012; 1251:E1–E24Crossref, Medline, Google Scholar
42 : Exposure to subliminal arousing stimuli induces robust activation in the amygdala, hippocampus, anterior cingulate, insular cortex and primary visual cortex: a systematic meta-analysis of fMRI studies. Neuroimage 2012; 59:2962–2973Crossref, Medline, Google Scholar
43 : Subliminal perception of others’ physical pain and pleasure. Exp Brain Res 2015; 233:2373–2382Crossref, Medline, Google Scholar
44 : The effect of context on responses to racially ambiguous faces: changes in perception and evaluation. Soc Cogn Affect Neurosci 2015; 10:885–892Crossref, Medline, Google Scholar
45 : Amygdala responsivity to high-level social information from unseen faces. J Neurosci 2014; 34:10573–10581Crossref, Medline, Google Scholar
46 : A review of neuroimaging studies of race-related prejudice: does amygdala response reflect threat? Front Hum Neurosci 2014; 8:179Crossref, Medline, Google Scholar
47 : Cultural specificity in amygdala response to fear faces. J Cogn Neurosci 2008; 20:2167–2174Crossref, Medline, Google Scholar
48 : Controlling racial prejudice: social-cognitive goals affect amygdala and stereotype activation. Psychol Sci 2005; 16:56–63Crossref, Medline, Google Scholar
49 : Inconsistencies in spontaneous and intentional trait inferences. Soc Cogn Affect Neurosci 2012; 7:937–950Crossref, Medline, Google Scholar
50 : The neural basis of stereotypic impact on multiple social categorization. Neuroimage 2014; 101:704–711Crossref, Medline, Google Scholar
51 : Dissociating bottom-up and top-down mechanisms in the cortico-limbic system during emotion processing. Cereb Cortex (Epub ahead of print Aug 27, 2014)Medline, Google Scholar
52 : The blame game: the effect of responsibility and social stigma on empathy for pain. J Cogn Neurosci 2010; 22:985–997Crossref, Medline, Google Scholar
53 : Empathy circuits. Curr Opin Neurobiol 2013; 23:275–282Crossref, Medline, Google Scholar
54 : Does context matter in evaluations of stigmatized individuals? An fMRI study. Soc Cogn Affect Neurosci 2013; 8:602–608Crossref, Medline, Google Scholar
55 : “Mental illness is like any other medical illness”: a critical examination of the statement and its impact on patient care and society. J Psychiatry Neurosci 2015; 40:147–150Crossref, Medline, Google Scholar
56 : Neurobiological narratives: experiences of mood disorder through the lens of neuroimaging. Sociol Health Illn 2013; 35:66–81Crossref, Medline, Google Scholar
57 : Effects of biological explanations for mental disorders on clinicians’ empathy. Proc Natl Acad Sci USA 2014; 111:17786–17790Crossref, Medline, Google Scholar
58 : Long-term reduction in implicit race bias: a prejudice habit-breaking intervention. J Exp Soc Psychol 2012; 48:1267–1278Crossref, Medline, Google Scholar



