Location of Acute Infarcts and Agitation and Aggression in Stroke
Abstract
The role of the infarct location in the development of poststroke agitation (PSA) is largely unknown. This study examined the association between the locations of infarcts and PSA at 9 months following the index stroke in 213 patients with the Chinese version of the Neuropsychiatric Inventory. Compared with the non-PSA group, PSA patients had a higher number and volume of acute pontine infarcts. Ventral pontine and lateral cerebellar infarcts were independent predictors of PSA in the multivariate analysis.
Agitation is a complex syndrome that comprises excessive motor activity (e.g., pacing or aimless wandering), verbal (e.g., complaining or repetitive sentences or questions), and physical aggression (e.g., spitting or hitting).1–3 Aggression is defined as a “complex behavior comprised of a composite of sensory, emotional, cognitive and motor elements,” in which behavior causes damage to individuals or property; attitudes, moods, or gestures are perceived as threatening or intimidating; and/or purposeful behavior disrupts rehabilitation activities and social reintegration.4 Agitation can occur with or without aggression and vice versa.1,5 Commonly used scales for the measurement of agitation include the Neuropsychiatric Inventory (NPI),6,7 the Cohen-Mansfield Agitation Inventory (CMAI),8 and the Behavioral Pathology in Alzheimer’s Disease (BEHAVE-AD).9 These scales do not clearly discriminate between agitation and aggression. The use of the composite term “agitation and aggression” in this article reflects their overlapping nature.
Poststroke agitation and aggression (PSAA) occurs in 15%−35% of all stroke survivors, assessed at 4 days to 12 months after stroke.10–18 The wide range of prevalence estimates reflects the heterogeneous definitions, differences in assessment methods, and varying times poststroke at which stroke survivors are assessed. With regard to definitional issues, some studies focus on agitation and aggression alone, while others extend the definition to include anger, hostility, and violence. For example, Paradiso et al.14 focused on “violent outbursts” (feelings of anger, quarreling, shouting, hitting people, and breaking things), Kim et al.13 used the Spielberger Trait Anger Index (STAI) to assess “inability to control anger or aggression,” and Chan et al.11 and Aybek et al.10 identified agitation as one component of “aggressiveness” using the Present State Examination and the Emotional Behavior Index, respectively. With regard to the phenomenology of poststroke agitation and aggression, two studies employed the NPI-12.12,17 Only the NPI-12 and PSE yield a severity score, the absence of which in many studies of this problem limits cross-study comparisons of symptom severity.
The frequency of PSAA ranges between 5.8% and 35% within 1 month10,14–16,18 and 15.7%−32% during 2–12 months after stroke.11–13,17 Several other factors could also contribute to the variance of prevalence figures reported in literatures, including time elapsed since the index stroke, location of stroke, and other sample characteristics. Using STAI and wide inclusion criteria (including psychiatric disorders and cognitive impairment), the rate of PSAA in postacute to chronic stroke was 32%,13 while strict inclusion criteria (without psychiatric disorders and cognitive impairment) and the same assessment instrument yielded only a 15.7% rate of PSAA in acute stroke.16 Only one study compared the NPI-agitation score of three groups of patients at 2, 6, and 12 months after stroke, respectively, with healthy controls.17 PSAA was significantly higher in patients at 2 months than in controls but not at the two later time points. However, PSAA frequency at each time point was not reported in this study, and the frequency of PSAA within the first year was 28%.17
PSAA is potentially harmful to patients, caregivers, and other patients. PSAA could lead to the breakdown of care and of much-needed supportive relationships.14 Clinical correlates of PSAA include young age,14 female sex,14 previous stroke,10 diabetes mellitus,16 severe stroke,16 cognitive impairment,14 depressive symptoms,14 and impaired physical functioning.14
A thorough search of the literature located only five brain imaging studies on PSAA.11–15 Radiological findings associated with PSAA are left hemisphere,14 anterior lesions,14 and lesions affecting the frontal,13 lenticulocapsular,13 and pontine base areas.13 Limitations of the above-mentioned brain imaging studies in PSAA include small sample size,12 variable timing of assessment, lack of standardized assessment of agitation, omission of important correlates of agitation such as depressive symptoms,15 mixing agitation with anger and hostility,15 and lack of detailed radiological examination.11,13–15 For instance, in the study by Paradiso et al.,14 only computer tomography (CT) was used as the imaging technique, while in the study by Kim et al.,13 a mixture of CT and magnetic resonance imaging (MRI) scans were used.
The lack of reports on the association between specific infarct locations and PSAA gave the impetus for this prospective study to examine to the locations of infarcts associated with PSAA at 9 months after the index stroke. PSAA was assessed using the Chinese version of the NPI-12, which has been has been extensively used to evaluate agitation in stroke,12,17 dementia,19 and other neurologic diseases.20 Based on the published MRI evidence on PSAA, associations between frontal, basal ganglia, and pontine infarcts and PSAA were hypothesized.
Methods
Patients
Of the 2,128 patients with first-ever or recurrent acute ischemic stroke who were admitted to the Acute Stroke Unit of the Prince of Wales Hospital in Hong Kong between November 2009 and June 2012, 1,291 received an MRI scan. All patients with an MRI scan were screened for the following inclusion criteria: 1) Chinese ethnicity; 2) Cantonese as the primary language; 3) age 17 years or older; 4) first or recurrent acute ischemic stroke occurring within 7 days prior to admission; and 5) ability and willingness to offer consent to participate in the study. The exclusion criteria were: 1) transient ischemic attack, cerebral hemorrhage, subdural hematoma, or subarachnoid hemorrhage; 2) history of a central nervous system disease apart from stroke; 3) history of depression or other psychiatric disorders (based on the medical record); 4) Mini-Mental State Examination (MMSE) score less than 2021; 5) severe aphasia (defined as a score of 2 or more in the best language item of the National Institutes of Health Stroke Scale [NIHSS]) or auditory or visual impairment; 6) physical frailty preventing participation in the interview; and 7) recurrence of stroke prior to the 9-month assessment. The study protocol was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong. All participants signed a consent form 3 months after the index stroke.
Collection of Demographic and Clinical Data
A research nurse from the Stroke Unit collected the demographic and clinical data and assessed stroke severity using the NIHSS22 within 2 days of admission. This nurse also contacted patients’ relatives. Data were stored in a stroke registry. A research assistant administered the MMSE, the Barthel Index (BI),23 and the 15–item Geriatric Depression Scale (GDS)24 9 months after the onset of the index stroke. GDS scores were treated as continuous variables.
Assessment of PSAA
The NPI, CMAI, and BEHAVE-AD are all widely used scales for screening and assessing agitation and aggression.1 However, the latter two have been mainly used in patients with cognitive impairments.8 Compared with these other semistructured interviews, interviewing a knowledgeable informant with the NPI takes less time and is relatively easy to complete. The NPI has five versions: NPI 10- and 12-item versions (NPI-10 and NPI-12, respectively), NPI-Nursing Home Version, NPI-Questionnaire (NPI-Q), and NPI-Clinician Version (NPI-C). NPI-Q contains only 12 screen questions, while NPI-12 includes 7–9 questions asked subsequent to each screening question. NPI-C is more updated, and it appears superior to NPI-12 in ascertaining agitation and aggression.25 Agitation and aggression are separated into two different subscales in the NPI-C, while they are combined into one subscale in the NPI-12. Since there is no validated Chinese version of NPI-C, the NPI-12 was adopted in this study.
Nine months after the onset of the index stroke, patients and their caregivers attended a research clinic where a psychiatrist (Y.K.T.) who was blind to the patients’ radiological data administered the agitation/aggression subscale of the Chinese version of the NPI-12.6,7 The NPI is based on a structured interview with a caregiver to determine the presence of agitation in the past month.7 The behavior must represent a distinct change from that shown by the patient before the stroke.26 PSAA was diagnosed if the respondent’s answer to the screening questions was “yes.” The screening questions were “Does the patient have periods when he/she refuses to let people help him/her? Is he/she hard to handle?” After a “yes” response, the following eight questions were asked: 1) “Does the patient get upset with those trying to care for him/her or resist activities such as bathing or changing clothes?” 2) “Is the patient stubborn, having to have things his/her way?” 3) “Is the patient uncooperative, resistive to help from others?” 4) “Does the patient have any other behavior that makes him hard to handle?” 5) “Does the patient shout or curse angrily?” 6) “Does the patient slam doors, kick furniture, throw things?” 7) “Does the patient attempt to hurt or hit others?” 8) “Does the patient have any other aggressive or agitated behavior?” The severity score in the scale ranges from 1 to 3, representing mild, moderate, and severe agitation/aggression, respectively.
Radiological Examination
MRI was performed with a 1.5-T system (Sonata, Siemens Medical, Erlangen, Germany) within 7 days of admission. The following sequences were acquired: diffusion-weighted imaging (DWI) (TR/TE/excitation=180/122/4, matrix=128×128, FOV=230 mm, slice thickness/gap=5 mm/1 mm, echo planar imaging, single-shot factor=90) with three orthogonally applied diffusion gradients (b values of 1,000, 500, and 0 seconds/mm2); axial spin echo (SE) T1-weighted FFE sequence (TR/TE/excitation=25/2.3/1, flip angle=30°, FOV=230 mm, slice thickness/gap=3 mm/−1.5 mm, matrix 256×256); turbo spin echo T2-weighted (TR/TE/excitation=2,622/80/2, turbo factor of 18, FOV=230 mm, slice thickness/gap=5 mm/0.5 mm, matrix=512×346); axial FLAIR [fluid attenuated inversion recovery] sequence (TR/TE/TI/excitation=11,000/125/2,800/1, turbo factor=31, FOV=230 mm, slice thickness/gap=5 mm/0.5 mm, matrix=352×248); and blood sensitive gradient echo sequence (TR/TE/excitation=350/30/2, flip angle=30°, slice thickness/gap=5 mm/0.5 mm, FOV=230 mm, matrix 256×256).
A neurologist (Y.K.C.) who was blind to the PSAA scores assessed all the MRIs. The number and size of acute infarcts affecting the frontal, temporal, parietal, and occipital lobes, subcortical white matter, thalamus, basal ganglia, brain stem, and cerebellum were evaluated. The total area of acute infarcts on the DWI was measured by the manual outlines of all areas with restricted water diffusion identified on the diffusion-weighted images with b values of 1,000. The total volume was calculated by multiplying the total area by the sum of the slice thickness and gap. Intrarater reliability tests were performed on 20 patients, with a good degree of agreement (volume of acute infarcts: kappa=0.96; number of infarcts: kappa=0.94). The severity of white matter hyperintensities (WMHs) on MRI was graded with the Fazekas scale at the periventricular and deep white matter regions using a scale ranging from 0 to 3.27 In this study, infarcts in frontal lobe, basal ganglia, pons, and cerebellum and deep white matter abnormality (DWMH) were further divided into subregions. Frontal lobe was divided into lateral, medial, and orbital and anterior cingulate regions; pons was divided into dorsal and ventral regions; cerebellum was divided into vermis and lateral regions; DWMH was divided into frontal, temporal, parietal, and occipital regions.
Statistical Analysis
The demographic and clinical variables and radiological characteristics of patients with PSAA (PSAA group) were compared with those without it (non-PSAA group). Continuous variables were analyzed using independent t tests or Mann Whitney U test, depending on the data distribution. Chi-square tests or Fisher’s exact test were used for categorical variables. Associative regression models were subsequently constructed. Risk factors with a value of p<0.05 were analyzed with multivariate logistic regression analysis using a forward stepwise selection strategy. Correlations between the characteristics of the frontal lobe infarcts were examined with Spearman’s rho. If the correlations between these characteristics were ≥0.50, then only one of them was entered into the regression model to avoid co-linearity. The alpha level was set at 0.05.
Results
Two hundred and thirteen patients met study entry criteria and formed the study sample (Figure 1). Patients who were excluded from the study had lower NIHSS scores (2.7±2.8 versus 3.8±3.8; p<0.001). There was no significant difference between the two groups in terms of age or sex distribution.
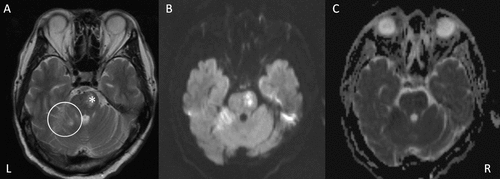
FIGURE 1. T2-Weighted MRIa
a The images show A) acute infarcts in the left-side ventral pons (asterisk) anterior lobe of the right cerebral hemisphere (circle), with corresponding high signal on B) diffusion-weighted imaging and low signal on C) apparent diffusion coefficient map. L=left; R=right.
Sixty (28.2%) of the 213 patients had PSAA. The demographic, clinical, and MRI characteristics of the whole sample and by two subgroups (PSAA/non-PSAA) are shown in Table 1. There were no differences between the PSAA and non-PSAA groups in age, sex, education level, history of hypertension, diabetes mellitus, or stroke, type of accommodation, and cohabitation status. PSAA patients were more likely to have a history of hyperlipidemia. There was no significant difference between the two groups in terms of stroke severity or cognitive functioning. The PSAA group had poorer physical functioning and more depressive symptoms.
| Characteristic | PSAA(N=60) | Non–PSAA(N=153) | p |
|---|---|---|---|
| Age (years) | 65.9±10.0 | 66.1±6.3 | 0.849b |
| Female sex | 22 (36.7%) | 68 (44.4%) | 0.301c |
| Education (years) | 6.4±3.8 | 6.4±4.2 | 0.829d |
| Cohabitation with family members | 136 (88.9%) | 55 (91.7%) | 0.819c |
| Living in institutions | 2 (1.3%) | 2 (3.3%) | 0.652e |
| Hypertension | 47 (78.3%) | 113 (73.9%) | 0.497c |
| Diabetes mellitus | 26 (43.3%) | 50 (32.7%) | 0.144c |
| Hyperlipidemia | 40 (66.7%) | 78 (51.0%) | 0.038c |
| Previous stroke | 6 (10.0%) | 8 (5.2%) | 0.225e |
| Antidepressant treatment after index stroke | 1 (1.7%) | 0 (0.0%) | 0.282e |
| NIHSS total score | 4.3±3.4 | 3.5±4.0 | 0.123d |
| BI score | 18.4±3.2 | 19.4±2.0 | 0.006d |
| GDS score | 5.1±4.2 | 2.6±2.8 | <0.001d |
| MMSE score | 27.6±2.7 | 27.6±2.6 | 0.996d |
| NPI-agitation/aggression severity | 3.0±2.2 | — | — |
TABLE 1. Demographic Characteristics, Psychosocial Risk Factors, and Clinical Characteristics by PSAA Statusa
All 60 PSAA patients had at least one positive response for the above eight questions. Fifty-five (91.7%) patients responded positively on question 1, 48 (80%) on question 2, 27 (45%) on question 3, 18 (30%) on question 5, seven (11.7%) on questions 4 and 6, four (6.7%) on question 7, and six (10%) on question 8. Nine patients had only one positive response, 19 had two, 16 had three, nine had four, three had five, three had six, and one had positive responses to all eight questions.
Patients with PSAA had a higher proportion of ventral pontine (21.7% versus 9.8%; p=0.021) and lateral cerebellar (11.7% versus 2.6%; p=0.019) acute infarcts and a lower proportion (6.7% versus 17.6%; p=0.041) of lateral prefrontal acute infarcts. The PSAA group also had a higher number (0.3±0.5 versus 0.1±0.4; p=0.004) and volume (0.2±0.5 versus 0.1±0.3 ml; p=0.007) of acute pontine infarcts. Lower, but not statistically significant, proportions of frontal and basal ganglia and higher, but not statistically significant, proportions of dorsal pontine acute infarcts and deep WMHs (DWMHs) were found in the PSAA group (Table 2). PSAA patients were more likely to have old infarcts (36.7% versus 22.9%; p=0.041). There was no difference between the two groups regarding white matter hyperintensities in subregions of deep white matter (Table 2). Lesion locations were similarly distributed in patients with higher or lower NPI agitation score.
| Characteristic | PSAA(N=60) | Non-PSAA(N=153) | p |
|---|---|---|---|
| Number of acute infarcts | 2.1±2.2 | 2.0±2.8 | 0.536c |
| Volume of acute infarcts (mm3) | 4.6±8.2 | 3.1±6.7 | 0.172c |
| Multiple lesions | 17 (28.3%) | 38 (24.8%) | 0.600b |
| Acute infarcts | |||
| Frontal | 5 (8.3%) | 27 (17.6%) | 0.087b |
| Later prefrontal | 4 (6.7%) | 27 (17.6%) | 0.041b |
| Medial prefrontal | 1 (1.7%) | 3 (2.0%) | 0.675d |
| Anterior cingulate | 1 (1.7%) | 1 (0.7%) | 0.920d |
| Orbital prefrontal | 0 (0%) | 0 (0.0%) | — |
| Temporal | 4 (6.7%) | 10 (6.5%) | 1.000d |
| Parietal | 2 (3.3%) | 11 (7.2%) | 0.360d |
| Occipital | 4 (6.7%) | 4 (2.6%) | 0.225d |
| Basal ganglia | 7 (11.7%) | 34 (22.2%) | 0.079b |
| Thalamus | 6 (10.0%) | 19 (12.4%) | 0.622b |
| Brain stem | 15 (25.0%) | 20 (13.1%) | 0.035b |
| Midbrain | 1 (1.7%) | 0 (0.0%) | 0.282d |
| Medulla | 0 (0.0%) | 5 (3.3%) | 0.325d |
| Pons | 15 (25.0%) | 15 (9.8%) | 0.004b |
| Dorsal | 4 (6.7%) | 2 (1.3%) | 0.096d |
| Ventral | 13 (21.7%) | 15 (9.8%) | 0.021b |
| Volume of pontine infarcts | 0.2±0.5 | 0.1±0.3 | 0.007c |
| Cerebellum | 7 (11.7%) | 4 (2.6%) | 0.019d |
| Vermis | 2 (3.3%) | 2 (1.3%) | 0.675d |
| Lateral | 7 (11.7%) | 4 (2.6%) | 0.019d |
| Subcortical white matter | 26 (43.3%) | 59 (38.6%) | 0.522b |
| Old infarcts | 22 (36.7%) | 35 (22.9%) | 0.041b |
| PVWMH score | 1.6±0.8 | 1.4±0.8 | 0.374c |
| DWMH score | 1.5±0.8 | 1.2±0.8 | 0.054c |
| Frontal WMH | 47 (78.3%) | 112 (73.2%) | 0.439b |
| Temporal WMH | 12 (20.0%) | 27 (17.6%) | 0.690b |
| Pariatal WMH | 35 (58.3%) | 92 (63.1%) | 0.810b |
| Occipital WMH | 18 (30.0%) | 42 (27.5%) | 0.710b |
TABLE 2. Stroke Severity and Radiological Characteristics by PSAA Statusa
The following six variables were entered into regression model A: presence of ventral pontine, lateral cerebellar, lateral frontal and basal ganglia acute infarcts, Fazekas score for DWMHs, and presence of old infarcts. Acute ventral pontine (odds ratio=2.814) and lateral cerebellar (odds ratio=6.738) infarcts and DWMH (odds ratio=1.569) were significant independent imaging predictors of PSAA. Hyperlipidemia, GDS, and BI scores were also entered into regression model B. The presence of acute ventral pontine and lateral cerebellar infarcts and DWMHs remained significant predictors of PSAA. The GDS score was another predictor. The R square of this model was 0.239 (Table 3). Acute ventral pons (χ2=0.810, Fisher’s exact p=0.450) and lateral cerebellum infarct (χ2=0.254, Fisher’s exact p=1.000) were equally distributed between patients with high (>8) or low (0–8) GDS scores.
| Variable | Model Ab | Model Bc | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | pd | Odds Ratio (95% CI) | pd | |
| Acute ventral pontine infarcts | 2.814 (1.211–6.539) | 0.016 | 3.363 (1.341–8.435) | 0.010 |
| Acute lateral cerebellar infarcts | 6.738 (1.806–25.144) | 0.005 | 7.747 (1.931–31.075) | 0.004 |
| DWMH score | 1.569 (1.078–2.285) | 0.019 | 1.576 (1.057–2.349) | 0.026 |
| GDS score | 1.228 (1.118–1.349) | <0.001 | ||
| Acute lateral frontal infarcts | 0.140 | 0.097 | ||
| Acute basal ganglia infarcts | 0.182 | 0.153 | ||
| Hyperlipidemia | 0.064 | |||
| Old infarcts | 0.494 | 0.232 | ||
| BI score | 0.279 | |||
| Nagelkerke R square | 0.113 | 0.239 | ||
TABLE 3. Multivariate Logistic Model of the Clinical Determinants of PSAAa
Discussion
To the best of our knowledge, this is the first MRI study to report associations between ventral pontine and lateral cerebellar infarcts, DWMH, and the risk of PSAA. The main strength of the study is the relatively large sample size and the detailed analysis of the MRI data. The main finding is that ventral pontine and lateral cerebellar infarcts are associated with an increased risk of PSAA in patients with well-established ischemic stroke.
No data have been published on the frequency of PSAA in Chinese stroke survivors. The frequency of PSAA in this study was in keeping within the 17%−35% range reported in Korean and Caucasian samples.10–16 Surprisingly, both the rate and severity of agitation/aggression are close to the 28.2% reported in one cross-sectional study,17 which also employed NPI-12 to assess agitation 1 year after stroke, with a mean agitation score of 1.5 at 2 months and slightly decreasing by 6 and 12 months. Patients' positive responses in both the present study and the above17 study were predominantly on the first four questions associated with agitation, indicating a higher frequency of agitation than aggression among poststroke patients.17 The inclusion criteria of the two studies are also compatible in that they included only patients with ischemic stroke, while those with history of psychiatric disorders and cognitive impairment were excluded. Lower rate of PSAA (15.7%) defined by NPI-12 was also reported at 3 months poststroke using the NPI-12.12 The reported lower PSAA frequency could be due to a less selective patient population within a shorter time following the index stroke.
The majority of the structural imaging studies on PSAA used rather crude divisions of lesion location, such as laterality12–14 or anterior/posterior.11,13–15 In contrast to our results, a Korean study found no association between pontine/cerebellum infarcts/DWMH and poststroke “anger proneness.”16 These investigators assessed patients 4.7 days after the onset of stroke, with “anger proneness” defined as an increased score on the 10-item Spielberger Trait Anger Scale. Hence, it is likely that these methodological differences play an important explanatory role in the diversity of findings reported here and previously.
The NPI has been criticized for lacking discriminating power between different disorders, for example, differentiating between depression and anxiety.20,25 In addition, factor analysis of the NPI agitation/aggression subscale indicates a variety of underlying symptoms and syndromes, ranging from hyperactivity28,29 to disinhibition,30 psychomotor disregulation,31 affective disturbances32 and even psychosis.33 In a recent study, the four NPI subscales—1) agitation/aggression, 2) irritability, 3) emotional lability, and 4) aberrant motor behavior and disinhibition)—were combined to measure the composite agitation phenomenon.34
The ventral pons comprises cortico-ponto-cerebellar projections that are from the sensorimotor, association, and paralimbic cortices to the cerebellum.35 Afferent information from these cerebral regions terminates on nuclei in ventral pons and then is conveyed from pons to cerebellum via mossy fiber.35 The cerebello-thalamic-cortical projections transfer feedback information from cerebellum to both motor and nonmotor cortical and subcortical regions. These cerebro-cerebellar loops provide structural substrate for the nonmotor function of cerebellum, such as modulating affective and cognitive functions.35 To support these assumptions, pontine lesions have been associated with uncontrollable anger, impulsion, irritability, and emotional incontinence.13,36,37
The contribution of the cerebellum to the modulation of human behavior and its role in the pathophysiology of neuropsychiatric disorders is gaining increasing attention.38,39 Cerebellar pathology is known to stimulate aggressiveness, irritability, and impulsivity in humans.40,41 Cortico-ponto-cerebellar pathology is associated with other discordant affectivity and behavior, such as apathy and emotional lability.13,36,42,43 Pontine and cerebellar infarcts can produce a distributed network effect, resulting in emotional dysregulation. In line with this vantage point, PSAA patients in this study also had higher depression scores than non-PSAA patients. The cortico-ponto-cerebellar pathology may be the common neurological substrate for both agitation and depression. Agitation co-occurring with depression is a feature of bipolar spectrum disorders44 and cerebellar damage.45 Patients with poststroke depression are more likely to have pontine microbleeds than their nondepressive counterparts.46 Reduced anterior cingulate-pons connection47 and cerebellum volume48 or function49 are also related to depression.
In this study, DWMHs were associated with PSAA. WMHs are correlated with neuropsychiatric symptoms in stroke with confluent white matter hyperintensities.50 White matter lesions may be related to agitation in other neurological disorders. Impaired deep white matter integrity is associated with increased agitation and irritability in mild cognitive impairment and Alzheimer’s dementia.51 Agitation is common in multiple sclerosis.52 Multifocal axonal varicosities and axonal loss have been found in the subcortical white matter in chronic traumatic encephalopathy, in which agitation is a characteristic symptom.53
The main limitation of this study is selection bias, as only a relatively small proportion of the original cohort of ischemic stroke patients was examined; this bias necessarily limits the generalizability of these findings. Another important limitation is the assessment of agitation 9 months following the index stroke, thereby narrowing the clinical relevance of the findings reported here to the chronic stage of stroke. Longitudinal studies have revealed that the frequency of other poststroke neuropsychiatric conditions, such as depression, varies at different poststroke time-points.4,10,11 Depression at subacute and postacute stroke may have different manifestations11 and etiologies,12 and the relationship between poststroke depression and PSAA may vary with time since stroke; accordingly, the relationship between these problems observed in our study may not hold in others, especially those performed at earlier or later times after stroke. Using the NPI agitation/aggression subscale to identify PSAA also may have falsely elevated the apparent prevalence of PSAA; for example, emotional lability, which may not be easily distinguished from agitation, may be an independent problem that once captured by this subscale, erroneously increases the apparent frequency of PSAA. Finally, depression may contribute to PSAA, although the analyses performed suggest that depressive symptoms do not alter the neuroanatomy of PSAA in this study sample.
Conclusions
Ventral pontine and lateral cerebellar infarcts are associated with a higher risk of PSAA and may contribute to its pathogenesis. Further investigations are warranted to clarify whether ventral pontine and lateral cerebellar infarcts have any effect on the clinical presentation, treatment response, and outcome of PSAA.
1 : Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr 2015; 27:7–17Crossref, Medline, Google Scholar
2 : Agitation: subtypes and their mechanisms. Semin Clin Neuropsychiatry 1996; 1:325–339Medline, Google Scholar
3 : Factor analysis of the Cohen-Mansfield Agitation Inventory in three large samples of nursing home patients with dementia and behavioral disturbance. Am J Geriatr Psychiatry 2005; 13:991–998Crossref, Medline, Google Scholar
4 : The agitated brain injured patient. Part 1: definitions, differential diagnosis, and assessment. Arch Phys Med Rehabil 1996; 77:617–623Crossref, Medline, Google Scholar
5 : Neurobiology of escalated aggression and violence. J Neurosci 2007; 27:11803–11806Crossref, Medline, Google Scholar
6 : Validation study of the Chinese version of the Neuropsychiatric Inventory (CNPI). Int J Geriatr Psychiatry 2001; 16:789–793Crossref, Medline, Google Scholar
7 : The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
8 : A description of agitation in a nursing home. J Gerontol 1989; 44:M77–M84Crossref, Medline, Google Scholar
9 : Scales to assess efficacy and safety of pharmacologic agents in the treatment of behavioral and psychological symptoms of dementia. J Clin Psychiatry 2001; 62(Suppl 21):19–22Medline, Google Scholar
10 : Emotional behavior in acute stroke: the Lausanne emotion in stroke study. Cogn Behav Neurol 2005; 18:37–44Crossref, Medline, Google Scholar
11 : Aggressive behavior in patients with stroke: association with psychopathology and results of antidepressant treatment on aggression. Arch Phys Med Rehabil 2006; 87:793–798Crossref, Medline, Google Scholar
12 : Premorbid personality traits are associated with post-stroke behavioral and psychological symptoms: a three-month follow-up study in Perth, Western Australia. Int Psychogeriatr 2009; 21:1063–1071Crossref, Medline, Google Scholar
13 : Inability to control anger or aggression after stroke. Neurology 2002; 58:1106–1108Crossref, Medline, Google Scholar
14 : Self-reported aggressive behavior in patients with stroke. J Nerv Ment Dis 1996; 184:746–753Crossref, Medline, Google Scholar
15 : Anger, hostility and aggression in the first days of acute stroke. Eur J Neurol 2006; 13:351–358Crossref, Medline, Google Scholar
16 : Factors associated with post-stroke anger proneness in ischaemic stroke patients. Eur J Neurol 2013; 20:1305–1310Crossref, Medline, Google Scholar
17 : Development of neuropsychiatric symptoms in poststroke patients: a cross-sectional study. Acta Psychiatr Scand 2004; 110:55–63Crossref, Medline, Google Scholar
18 : Subjective experience and behavior in acute stroke: the Lausanne Emotion in Acute Stroke Study. Neurology 1999; 52:22–28Crossref, Medline, Google Scholar
19 : Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348Crossref, Medline, Google Scholar
20 : The merits and problems of Neuropsychiatric Inventory as an assessment tool in people with dementia and other neurological disorders. Clin Interv Aging 2014; 9:1051–1061Crossref, Medline, Google Scholar
21 : Reliability and validity of the Cantonese version of the Mini-Mental State Examination: a preliminary study. Hong Kong J Psychiatry 1994; 4:25–28Google Scholar
22 : Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20:864–870Crossref, Medline, Google Scholar
23 : Functional evaluation: the Barthel index. Md State Med J 1965; 14:61–65Medline, Google Scholar
24 : Validation and comparison of three brief depression scales in an elderly Chinese population. Int J Geriatr Psychiatry 2000; 15:824–830Crossref, Medline, Google Scholar
25 : The Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr 2010; 22:984–994Crossref, Medline, Google Scholar
26 : Caregiver burden associated with behavioral and psychological symptoms of dementia in elderly people in the local community. Dement Geriatr Cogn Disord 2007; 23:219–224Crossref, Medline, Google Scholar
27 : MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 1987; 149:351–356Crossref, Medline, Google Scholar
28 : Behavioral problems in dementia: a factor analysis of the neuropsychiatric inventory. Dement Geriatr Cogn Disord 2003; 15:99–105Crossref, Medline, Google Scholar
29 : Grouping of behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry 2014; 29:562–568Crossref, Medline, Google Scholar
30 : Behavioral syndromes in Alzheimer’s disease. Int Psychogeriatr 1992; 4(Suppl 2):161–184Crossref, Medline, Google Scholar
31 : Behavioral disorders and caregivers’ reaction in Taiwanese patients with Alzheimer’s disease. Int Psychogeriatr 2001; 13:121–128Crossref, Medline, Google Scholar
32 : Behavioural and psychological syndromes in Alzheimer’s disease. Int J Geriatr Psychiatry 2004; 19:1035–1039Crossref, Medline, Google Scholar
33 : Behavioral syndromes in Alzheimer’s disease: description and correlates. Dement Geriatr Cogn Disord 1999; 10:130–138Crossref, Medline, Google Scholar
34 Frontolimbic atrophy is associated with agitation and aggression in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement 2013; 9:S95–S104Crossref, Medline, Google Scholar
35 : Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 2010; 46:831–844Crossref, Medline, Google Scholar
36 : Pathoanatomic correlation between poststroke pathological crying and damage to brain areas involved in serotonergic neurotransmission. Stroke 1994; 25:1050–1052Crossref, Medline, Google Scholar
37 : Transient abnormal behavior after pontine infarction. Stroke 1994; 25:2295–2296Crossref, Medline, Google Scholar
38 : The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum 2007; 6:254–267Crossref, Medline, Google Scholar
39 : An emerging concept. The cerebellar contribution to higher function. Arch Neurol 1991; 48:1178–1187Crossref, Medline, Google Scholar
40 : Impulsive behavior and recurrent major depression associated with Dandy-Walker variant. Psychiatry Investig 2013; 10:303–305Crossref, Medline, Google Scholar
41 : Hereditary ataxia and behavior. Adv Neurol 2005; 96:275–283Medline, Google Scholar
42 : The cerebellar cognitive affective syndrome. Brain 1998; 121:561–579Crossref, Medline, Google Scholar
43 : Pure post-stroke cerebellar cognitive affective syndrome: a case report. Neurol Sci 2004; 25:220–224Crossref, Medline, Google Scholar
44 : Agitated depression: a valid depression subtype? Prog Neuropsychopharmacol Biol Psychiatry 2004; 28:1279–1285Crossref, Medline, Google Scholar
45 : Bipolar spectrum disorders in patients with cerebellar lesions: a comparison with Parkinson’s disease. J Nerv Ment Dis 2015; 203:725–729Crossref, Medline, Google Scholar
46 : Pontine microbleeds and depression in stroke. J Geriatr Psychiatry Neurol 2014; 27:159–164Crossref, Medline, Google Scholar
47 : Altered functional connectivity between medial prefrontal cortex and the inferior brainstem in major depression during appraisal of subjective emotional responses: a preliminary study. Biol Psychol 2015; 108:13–24Crossref, Medline, Google Scholar
48 : Computational meta-analysis of statistical parametric maps in major depression. Hum Brain Mapp 2016; 37:1393–1404Crossref, Medline, Google Scholar
49 : The role of cerebellum in unipolar and bipolar depression: a review of the main neurobiological findings. Riv Psichiatr 2014; 49:124–131Medline, Google Scholar
50 : Executive dysfunction and left frontal white matter hyperintensities are correlated with neuropsychiatric symptoms in stroke patients with confluent white matter hyperintensities. Dement Geriatr Cogn Disord 2010; 30:254–260Crossref, Medline, Google Scholar
51 : Diffusion tensor imaging of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s dementia. J Neuropsychiatry Clin Neurosci 2012; 24:484–488Link, Google Scholar
52 : Neuropsychiatric manifestations of multiple sclerosis. J Neuropsychiatry Clin Neurosci 1999; 11:51–57Link, Google Scholar
53 : The spectrum of disease in chronic traumatic encephalopathy. Brain 2013; 136:43–64Crossref, Medline, Google Scholar



