Psychiatric Morbidity and Its Prognosis in Posterior Reversible Encephalopathy Syndrome
Abstract
Posterior reversible encephalopathy syndrome (PRES) is a clinically and radiologically diagnosed disorder distinguished by subcortical vasogenic cerebral edema. To date, its presentation has been described through summarized neurological categories, such as seizures, headaches, “confusion,” and “altered mental function.” This retrospective case series identified all cases of clinically confirmed, radiologically diagnosed PRES resulting in treatment in a large teaching hospital from 2010 to 2019. The authors conducted a search for the term “reversible encephalopathy” in the hospital clinical radiology information system, followed by an audit of scan reports and clinical records. The most common reasons for psychiatric referral were addictions, acute psychosis, depression, suicidality, and treatment refusal. Multidisciplinary staff should consider PRES as a rare, organic differential diagnosis for acute mental state changes. Physicians should be aware of elevated rates of post-PRES psychiatric symptoms and consider whether psychiatric consultation may enhance recovery.
Posterior reversible encephalopathy syndrome (PRES) is clinically and radiologically diagnosed and characterized by subcortical vasogenic cerebral edema, with characteristic neuroimaging appearances. Its commonest known precipitants are drug toxicity (1), hypertension, sepsis (2), preeclampsia, and autoimmune disease. In a review of the literature of the past 20 years, Gao et al. (3) highlighted unanswered questions about the underlying pathophysiology, diagnostic criteria, and prognosis of PRES. The importance of clinical suspicion has been emphasized (4); however, presenting symptom descriptions have been confined to summarized neurological categories, such as seizures, headaches, “confusion,” and “altered mental function” (5–7). Studies of the psychiatric sequelae of PRES and its mental health comorbidity are, to our knowledge, absent from the literature.
Following the description of new neuropsychiatric syndromes, such as anti-N-methyl-d-aspartate receptor encephalitis (8), interest in potentially reversible “organic” etiologies of psychiatric presentations has expanded markedly (9, 10). Research on psychiatric outcomes of acute neurological disorders is well established, but recent work on encephalitides is noteworthy, reporting high rates of anger, anxiety, mood swings, and low mood (11). For example, a large population-based retrospective cohort study revealed elevated risk ratios for bipolar affective disorder (risk ratio=6.34) as well as for psychotic (risk ratio=3.48), depressive (risk ratio=1.88), and anxiety (risk ratio=1.46) disorders postencephalitis (12). Comorbid psychiatric disorders are associated with worse quality of life in a range of neurological disorders, including multiple sclerosis (13), epilepsy (14), and migraine (15).
Based on clinical experience, we hypothesized that a proportion of patients diagnosed with PRES would develop psychiatric symptoms during and after the acute illness. We aimed to identify all cases of PRES radiologically diagnosed in a large teaching hospital over a 10-year period to audit psychiatric precipitants, symptoms, and sequelae, alongside clinical and etiological correlates.
Methods
In a retrospective case series conducted in the liaison neuropsychiatry service of a large South London teaching hospital serving four boroughs, we identified patients with PRES presenting between April 2010 and April 2019. The study was authorized by the South London and Maudsley National Health Service Foundation Trust Clinical Audit and Effectiveness Team on June 16, 2016. We retrieved records from the hospital’s clinical radiology information system using the search term “reversible encephalopathy” to address disambiguation problems with “PRES.” For each patient for whom a scan report mentioned reversible encephalopathy, we reviewed clinical and radiological records to determine whether PRES was ultimately the confirmed diagnosis. We audited the clinical records of these patients using a piloted data collection form and entered pseudonymized data into a spreadsheet stored in password-protected hard drives.
Results
Case Detection
Of an initial 122 results, 69 patients were excluded at initial screening (duplicates, N=18; no scan recorded, N=13; and PRES ruled out radiologically, N=38; Figure 1). On the basis of 53 clinical notes reviewed, we excluded seven cases in which the ultimate clinical or radiological impression was not PRES. Forty-seven case subjects were included in the final study sample, including one whose diagnosis was reported by the neurology team.
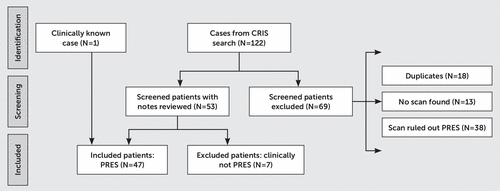
FIGURE 1. Flow diagram of study subjects with posterior reversible encephalopathy syndrome (PRES)a
a CRIS=clinical radiology information system.
Demographic Characteristics
Of the 47 included case subjects, 33 (70%) were female, with a median age of 39 years at presentation (range, 5–74 years).
Neuroimaging
Radiological reports revealed that abnormalities were bilateral in 44 (94%) case subjects; of these, 25 (57%) were asymmetrical. Gray matter involvement was mentioned in 23 (49%) reports, and abnormalities were more pronounced in posterior regions compared with anterior regions in 40 patients (85%). Involved brain regions were occipital (N=40; 85%), parietal (N=37; 79%), frontal (N=20; 43%), temporal (N=17; 36%), cerebellar (N=12; 26%), basal ganglia (N=7; 15%), and brainstem (N=4; 9%).
Presenting Symptoms
The most common presenting symptoms were seizures (N=38; 81%), reduced consciousness (N=29; 62%), headache (N=12; 26%), visual abnormalities (N=12; 26%), and nausea and vomiting (N=11; 23%). Focal neurological signs were documented for 10 (21%) patients, with confusion and agitation present in seven (15%) cases each.
Etiology
Acute hypertension was identified in 34 (72%) patients. When blood pressure at the time of presenting symptoms was electronically documented, the mean systolic pressure was 185 mmHg (SD=24; range, 140–234 mmHg; N=22), and the mean diastolic pressure was 109 mmHg (SD=19; range, 71–140 mmHg; N=17). Possible drug toxicity was identified in 27 (57%) patients, including for tacrolimus (N=8; 30%), cyclosporine (N=6; 22%), and a range of other agents, which included cancer chemotherapeutic agents (N=4; 15%), anti-inflammatory drugs (N=4; 15%), and antibiotics (N=2; 7%). Infection or sepsis was present in 22 (47%) patients; of these cases, 10 (46%) involved chest sepsis, and each of three (14%) involved both HIV opportunistic infections and skin infections, while other cases involved bacterial infections, including clostridium difficile diarrhea (N=2).
Medical Comorbidities
The commonest medical comorbidities were hematological (N=14; 30%), including aplastic anemia (N=3), acute leukemias (N=3), myelodysplastic syndrome (N=2), and sickle cell anemia (N=2). The second most common comorbidities were autoimmune disorders (N=13; 28%), including systemic lupus erythematosus (N=5; 38%), sarcoidosis (N=2; 15%), and autoimmune hepatitis (N=2; 15%). Chronic liver disease affected 11 (23%) patients, while eight (17%) patients had chronic kidney disease, with seven (88%) requiring renal replacement therapy. Seven patients were diagnosed with malignancy (15%), and four (9%) had a history of seizures. Three PRES cases (6%) occurred during a pregnancy or postpartum period.
Management
The most frequently prescribed treatments were antiepileptic medications (N=13; 27%), antihypertensive medications (N=13; 27%), or a combination of both (N=8; 17%). Five patients (11%) received treatment for potential CNS infection, and in 11% of these, no specific treatment for PRES was provided beyond management of comorbid disorders or withdrawal of the suspected toxic agent.
Prognosis
Seven patients (15%) died during the index hospital admission, one patient was discharged to palliative care, and five patients died after the index hospital admission, resulting in a mortality rate of 28% during the follow-up period. Age of death ranged from 5 to 74 years old, with a median age of 41 years.
Psychiatric Contact
Eighteen (38%) case subjects had some form of contact with local psychiatric services. This subgroup of patients had a higher median age (44 years old versus 39 years old), a higher proportion of females (83% versus 70%), and a higher mortality rate (33% versus 28%) compared with the total sample. Psychiatric services contact comprised pre-PRES consultation (N=7; 15% of all cases), liaison psychiatry referrals during the index admission (21%), and post-PRES consultation (21%). The median duration of psychiatric services consultation from PRES onset until hospital discharge was 677 days (range, 7–3,123 days).
Premorbid Conditions
Reasons for psychiatric treatment before PRES diagnosis were assessments of low mood and suicidality (29%), regular consultation from addictions services (29%), mental capacity assessments due to dialysis refusal (29%), and routine assessment pretransplant surgery (14%).
Psychiatric PRES Symptoms
Mental state abnormalities were documented in 12 PRES case records (26%). These abnormalities were speech disturbance (67%), confusion (58%), agitation (58%), hallucinations (33%), disinhibition (25%), low mood (25%), delusions (17%), bad or vivid dreams (17%), and religious preoccupation, self-harm, and anxiety (8%). Of the 10 patients who were referred to liaison psychiatry, only one was evaluated for symptoms directly related to PRES, and three were evaluated for symptoms identified once PRES had been treated. The remaining referrals were for medication overdoses that precipitated hospital admission (20%), cognitive or mental capacity assessment (20%), or substance-use nurse review (20%). The one patient who was referred to psychiatric services during PRES onset and treatment was evaluated for grandiose and persecutory delusions and auditory hallucinations. The three patients who were evaluated for post-PRES symptoms were referred for agitated depression and suicidality, low mood and vivid dreams, and advice regarding antipsychotic medication.
Psychiatric Symptoms Post-PRES Hospital Admission
Of the 10 patients who received psychiatric services post-PRES hospital admission, four were referred for low mood, along with acute delusions, suicidality, erratic behavior, or reduced oral intake. In addition, four patients were referred regarding their capacity to refuse treatment or self-discharge; of these, comorbid addictions were present in three. Two patients were referred for acute confusion, along with persecutory delusions or rapid cognitive decline.
Discussion
In this study, we found that psychiatric symptoms were not reported in all cases of PRES, although confusion and agitation were common. However, psychiatric symptoms often occurred before, during, or following PRES onset, which is consistent with evidence of psychiatric comorbidities in neurological disorders, including epilepsy, migraine, stroke, and traumatic brain injury (16). The subgroup of patients who received psychiatric consultation was a median of 5 years older than the total sample, with a higher proportion of females and a higher mortality rate (33% versus 28%).
Physicians treating PRES and liaison psychiatrists should be alert to psychiatric comorbidities. In addition, multidisciplinary staff should consider PRES as a rare, organic differential diagnosis for acute mental state changes, especially when PRES coincides with neurological symptoms, hypertension, or medical comorbidities. Physicians treating PRES should assess for post-PRES psychiatric symptoms, to consider whether consultation-liaison psychiatry, specialist substance use, or community psychiatric follow-up may enhance recovery.
Given these rates of psychiatric comorbidity, it is striking that mental health comorbidity is near-absent from literature on PRES, which focuses on neuroimaging, neurological sequelae, treatment, and prognosis. This is surprising, given the association between neuropsychiatric symptoms and temporo-limbic network lesions, which are not uncommon in PRES. Despite the term reversible, residual infarcts and subsequent leukomalacia are recognized sequelae of PRES (17), supporting the likelihood of longer-term psychiatric symptoms in a proportion of patients, which is well recognized in acute neurological disorders such as encephalitis (11, 12).
As expected, our study sample had high rates of underlying medical comorbidity. Worldwide, depression prevalence is higher among people with chronic conditions (18), including neurological (19) disorders. The most common reasons for psychiatric services referral in our sample were alcohol and intravenous drug addictions, acute psychosis, depression, suicidality, and refusal of medical treatment due to delirium or agitation. There were few or no referrals for severe anxiety, long-standing psychotic illnesses, bipolar illness, intellectual disability, and personality or eating disorders. Perhaps during critical illness treatment and follow-up, only markedly acute psychiatric symptoms were detected by physicians and referred for assessment. It is not possible to definitively quantify the extent to which some psychiatric morbidity predating PRES onset may have gone undetected. Such morbidity would be expected to be increased over the population baseline in the context of chronic medical illness. The United Kingdom National Health Service provides open access to family health practitioners, and therefore most moderate to severe mental disorders are recorded. Substance use disorders, however, may not always be identified, treated, and recorded by health care services.
To our knowledge, this is the first study to provide preliminary support for our clinically informed hypothesis of elevated psychiatric morbidity among individuals following PRES onset, which requires more widespread, prospective investigation. One strength of our study is its relatively large sample size, drawn from a broad, diverse, densely populated region of South London in a teaching hospital with expertise in a range of relevant medical disorders. One limitation is its pragmatic, retrospective audit design, dependent on routinely documented free-text clinical records. Future research and PRES case series should investigate psychiatric comorbidity and sequelae systematically, including the premorbid period, to elaborate on these findings. Perspectives of patients and their care givers on pre- and post-PRES mental states, as well as longitudinal follow-up of neuropsychiatric outcomes, would be particularly informative.
1 : Tacrolimus neurotoxicity and the role of the Renin-Angiotensin system. J Neuropsychiatry Clin Neurosci 2015; 27:e140–e141Link, Google Scholar
2 : Sepsis-associated encephalopathy: review of the neuropsychiatric manifestations and cognitive outcome. J Neuropsychiatry Clin Neurosci 2011; 23:237–241Link, Google Scholar
3 : Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? J Neurol Neurosurg Psychiatry 2018; 89:14–20Crossref, Medline, Google Scholar
4 : Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 2015; 14:914–925Crossref, Medline, Google Scholar
5 : Understanding Posterior Reversible Encephalopathy Syndrome, in Annual Update in Intensive Care and Emergency Medicine. Edited by Vincent JL. Berlin, Springer, 2011, pp 631–653Google Scholar
6 : Posterior reversible encephalopathy syndrome. Curr Opin Neurol 2019; 32:25–35Crossref, Medline, Google Scholar
7 : Posterior reversible encephalopathy syndrome: long-term follow-up. J Neurol Neurosurg Psychiatry 2010; 81:773–777Crossref, Medline, Google Scholar
8 : Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008; 7:1091–1098Crossref, Medline, Google Scholar
9 : Study of immunotherapy in antibody positive psychosis: feasibility and acceptability (SINAPPS1). J Neurol Neurosurg Psychiatry 2019; 90:365–367Crossref, Medline, Google Scholar
10 : Emerging psychiatric syndromes associated with antivoltage-gated potassium channel complex antibodies. J Neurol Neurosurg Psychiatry 2016; 87:1242–1247Crossref, Medline, Google Scholar
11 : Consequences of encephalitis. Malton, United Kingdom, Encephalitis Society, 2000Google Scholar
12 : Increased rates of sequelae post-encephalitis in individuals attending primary care practices in the United Kingdom: a population-based retrospective cohort study. J Neurol 2017; 264:407–415Crossref, Medline, Google Scholar
13 : Depression and quality of life in multiple sclerosis. Acta Neurol Scand 2001; 104:257–261Crossref, Medline, Google Scholar
14 : The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia 2004; 45:544–550Crossref, Medline, Google Scholar
15 : Migraine and psychiatric comorbidity: a review of clinical findings. J Headache Pain 2011; 12:115–125Crossref, Medline, Google Scholar
16 : Management of psychiatric and neurological comorbidities in epilepsy. Nat Rev Neurol 2016; 12:106–116Crossref, Medline, Google Scholar
17 : Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol 2008; 65:205–210Medline, Google Scholar
18 : Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858Crossref, Medline, Google Scholar
19 : Comorbidity between neurological illness and psychiatric disorders. CNS Spectr 2016; 21:230–238Crossref, Medline, Google Scholar



