Deep Brain Stimulation for OCD in a Patient With Comorbidities: Epilepsy, Tics, Autism, and Major Depressive Disorder
Obsessive-compulsive disorder (OCD) is a debilitating psychiatric condition found in 2.3% of the U.S. population (1). OCD is most often comorbid with other disorders (91% lifetime disorders and 42% current disorders), such as major depressive disorder (MDD; 35%−67% of people with OCD also have MDD) (2) and tic disorder (20%−30%) (3). Individuals with OCD are 13 times more likely than the general population to have autism spectrum disorder (ASD; 6.6% versus 0.5%) (4). Obsessive-compulsive symptoms are found in 30.8% of people with Tourette’s syndrome and in 81.3% of people with ASD (5). Individuals with comorbid tic disorder or ASD may have OCD illness that is particularly difficult to treat (6, 7).
The gold-standard treatment for OCD, with or without comorbid conditions, is exposure and response prevention, alone or in combination with serotonergic medication. Even with optimal treatment, only 35% of individuals with OCD achieve remission (defined as a Yale-Brown Obsessive Compulsive Scale [Y-BOCS] score in the subclinical range, ≤7) (8), and about 10% of patients remain severely impaired (9). One recent development for treating refractory OCD is deep brain stimulation (DBS).
Not yet a common procedure for the treatment of OCD, DBS was approved by the Food and Drug Administration (FDA) in 2009 as a humanitarian use device under a humanitarian device exemption for the treatment of refractory severe to extreme OCD. The approved anatomical target is the anterior limb of the internal capsule (ALIC), with the tip of the electrode in the ventral capsule/ventral striatum. Other targets investigated in OCD include the nucleus accumbens (which can be targeted with the electrode passing through the ALIC), subthalamic nucleus, inferior thalamic peduncle, bed nucleus of stria terminalis, and the superolateral branch of the median forebrain bundle (10). The 2009 approval of DBS for intractable OCD underscores the likely efficacy of DBS in treating severe, refractory OCD while acknowledging that routine use is not advisable due to the absence of large randomized controlled trials, which are in part lacking due to concerns about the vulnerability of psychiatric patients and the cautionary history of early psychosurgery. Candidates must have a thorough psychiatric evaluation that includes suicide risk assessment by a psychiatrist who is an expert in the disorder being treated by DBS. The DBS evaluation and procedure must be done within the context of a multidisciplinary team that includes a stereotactic and functional neurosurgeon, a psychiatrist, a neurologist, and a neuropsychologist (11).
Unfortunately, very little is known about how DBS affects individuals with OCD who also have other psychiatric and neurological comorbid conditions (12–15). The FDA-approved ALIC target used in OCD is not the location most often targeted in Tourette’s syndrome, but it is part of the same neurocircuitry that is aberrant in Tourette’s syndrome. Targets that have been investigated for Tourette’s syndrome include the thalamus, globus pallidus, Forel’s field H1, subthalamic nucleus, and nucleus accumbens/ALIC (16). Not surprisingly, there is limited research that suggests comorbid OCD symptoms also improve when these other anatomical locations are stimulated (16). Other comorbid symptoms such as depression, anxiety, or substance misuse may improve as well (16). A few case studies have demonstrated reduction in both tics and OCD symptoms with stimulation of the nucleus accumbens/ALIC (13, 17). Combined DBS of globus pallidus internus and capsulotomy has been reported to treat symptoms of both OCD and Tourette’s syndrome (18).
It is important to note that current DSM-5 diagnostic criteria attempt to lump sets of symptoms into discrete disorders; in reality, this patient likely has a particular pattern of brain dysfunction and aberrant neurocircuitry that contributes to the entirety of his particular phenotype (19), referred to in this paper as comorbid disorders. We present the case of a middle-aged man with refractory OCD as well as epilepsy, tic disorder, autism, and MDD, and the interactions and results of DBS on all four disorders.
Case Report
The patient, a 44-year-old man with OCD, MDD, autism, and tics, was evaluated for potential DBS for treatment-refractory OCD. His symptoms did not improve with multiple pharmacologic and psychotherapeutic treatments, and he struggled with OCD for more than 20 years. His medication regimen consisted of fluvoxamine (200 mg daily), risperidone (2.5 mg daily), N-acetylcysteine, and carbamazepine (for controlled epilepsy), a regimen with potential for significant drug interactions. Despite treatment, his Y-BOCS scores over the course of 17 months prior to surgery ranged from 36 to 39, which is consistent with severe OCD.
The patient’s OCD symptoms began when he was in his twenties, with a need for things in his life to feel “just right,” along with excessive handwashing and checking behaviors. Because of these symptoms, he left college and moved in with his mother. By age 40, numerous obsessions and compulsions consumed most of his day.
The patient’s presentation was further complicated by high-functioning autism, tics, and MDD. His autism, formally diagnosed at age 40, was characterized by difficulty interpreting and responding to social cues and emotions of others, unusual prosody of speech, limited or irregular use of nonverbal communication, inability to form or maintain relationships with people other than his mother, and a fixed interest in football and game statistics, preceded in childhood by a fixed interest in dinosaurs. Although it seemed likely that delayed recognition was the reason for late diagnosis in this patient, it is important to consider that several infectious or autoimmune etiologies, such as herpes encephalitis and anti-N-methyl-d-aspartate-receptor encephalitis, can lead to symptoms similar to autism with other neuropsychiatric sequelae, including seizures (20, 21).
The patient’s tics were difficult to disentangle from his OCD. This is important because, as noted above, the ALIC target approved for OCD is not typically targeted in Tourette’s syndrome (16). The patient’s motor and phonic tics included frequent blinking and hard rubbing of his eyes, staring, poking, and repetitive coughing and throat clearing combined with violent, jerky head and neck movements. These movements were performed until they felt “right” or were executed in defined number combinations. They were accompanied by a premonitory sensation or physical urge to perform the movements, and sometimes they were done to prevent something bad from happening. The patient’s tics were late in onset, beginning between ages 35 and 37; thus, his official DSM-5 diagnosis was most consistent with “other specified tic disorder, onset after age 18.” After much discussion, our team concluded that his presentation was most consistent with tic-related OCD or Tourettic OCD, in which tics are intimately combined with OCD (22). Although Tourettic OCD is not a formal DSM-5 diagnosis, this formulation captures the overlapping phenomenology sometimes found in tics and compulsions.
The patient experienced the onset of seizures at age 18, described as an aura of impaired concentration, followed by anxiety and loss of consciousness with whole-body convulsions. He remained untreated for 15 years and had approximately 15 seizures during this period. Subsequently, he was started on phenytoin but was switched to carbamazepine shortly thereafter. He had been seizure-free for the past 17 years. Evaluations, including EEG and MRI at age 44, were unremarkable.
The patient’s symptoms had a deleterious effect on his quality of life. He lived with his mother, whose help he needed with the most basic activities of life, such as leaving his room to eat meals. He had been unable to complete college, despite good performance and advanced-placement classes in high school, and he had received Social Security Disability Income for mental illness since age 29. He was only able to work 1 day a week in a janitorial capacity. His checking rituals also interfered significantly with basic activities, such as grooming and bathing.
The patient and his mother initially participated in a clinical interview with diagnostic rating scales, including the Y-BOCS and Montgomery-Å'sberg Depression Rating Scale (MADRS). Because no validated rating scale assesses Tourettic OCD, the Yale Global Tic Severity Scale (YGTSS) was used to evaluate motor and phonic symptoms. The same battery of diagnostic rating scales (except the YGTSS) was administered two more times prior to DBS surgery and at programming visits following surgery. The patient’s self-reports and self-observations indicated far fewer symptoms and impairments than those reported by his mother or perceived by his clinicians. These discrepancies were possibly caused by his difficulties in accurately assessing and describing his inner emotional states, attributable to comorbid autism, as well as to his strong desires to appear well and avoid stigmatizing shame. In order to accurately rate symptoms on clinician-rated scales, discussions involved both the patient and his mother until a consensus was reached.
Consequent to the surgical team’s expressed concerns about the patient’s capacity to consent to DBS, an independent capacity evaluation was performed by a psychiatrist who was not otherwise involved in the patient’s assessment or treatment. This evaluation revealed that the patient initially had unrealistic expectations for surgery, because he expressed hope that surgery would help him return to a normal life. In collaboration with his primary psychiatrist, his therapist, and his mother, we provided psychoeducation to help mitigate expectations prior to surgery. We made it clear that DBS of the ventral capsule/ventral striatum could not be expected to reduce the aspects of his disability associated with autism.
At age 46, he underwent MRI-guided asleep surgery using the ClearPoint Neuro Navigation System to place bilateral 4-contact electrodes (Model 3391, Medtronic, Minneapolis) in the ventral capsule/ventral striatum, followed by a second surgery to place Medtronic Activa SC implantable neurostimulators. Postoperative imaging confirmed lead location, with the most ventral contacts located in the region of the nucleus accumbens. After surgery, the patient’s Y-BOCS and MADRS scores decreased by 68% and 66%, respectively (relative to averaged presurgical scores) (Figure 1A). These dramatic reductions were likely caused by a microlesion effect, commonly seen in DBS for Parkinson’s disease as a transient improvement in symptoms even prior to stimulation activation (23). This effect is a result of cell injury and tends to resolve over the course of several weeks. The DBS programming algorithm described by Widge and Dougherty (24) was followed, with adjustments to the algorithm as needed. Selection of parameters was guided by assessment of the patient’s anxiety, energy, mood, and subjective experience and the programmer’s observations of affect and engagement.
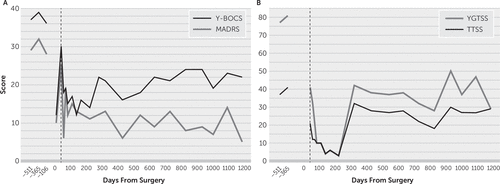
FIGURE 1. Interactions and results of deep brain stimulation (DBS) in a middle-aged patient with refractory obsessive-compulsive disorder (OCD) comorbid with epilepsy, tic disorder, autism, and major depressive disordera
a Panel A shows the severity scores for OCD (with the Yale-Brown Obsessive Compulsive Scale [Y-BOCS]) and depression (with the Montgomery-Ǻsberg Depression Rating Scale [MADRS]) assessed pre-DBS and post-DBS surgery. Panel B shows the Total Tic Severity Score (TTSS) and Yale Global Tic Severity Scale (YGTSS) score assessed pre-DBS and post-DBS surgery. Presurgery values are not to scale. The dotted line indicates stimulation activated 42 days postsurgery.
After 6 weeks of programming, the patient reported marked reduction in OCD symptoms and depression, and his scores on the Y-BOCS and MADRS indicated that his symptoms were in the mild range. Over the past 3 years, the patient’s Y-BOCS scores indicated symptom fluctuation between mild and severe, with his most recent score representing a 41% score reduction since surgery. The patient’s average Y-BOCS score reduction 6 months to 37 months poststimulation was 49%. We hypothesized that the earlier increase in his Y-BOCS score was due in part to his symptoms becoming more obvious to him and his mother as his level of functioning increased and as he became more engaged in life. For example, when he attended school, he became more aware of the impairment, caused by his symptoms, related to test-taking and being on time to class (which he did not notice when he was at home all of the time). This impairment was reflected in his rating scale scores. His depression score was well below pre-DBS levels (Figure 1A).
In addition, the patient’s motor and phonic symptoms decreased markedly. He had a 50% reduction in his YGTSS score on the day before DBS was turned on (likely due to microlesion effect) and further reduction (75%) 6 weeks after DBS was activated (Figure 1B). He was least symptomatic 6 months after stimulation was turned on (with 96% reduction in his YGTSS score). His YGTSS scores continued to indicate symptom improvement, although scores for both YGTSS and tics increased 9 months after surgery, and this increase has persisted. His most recent YGTSS score indicated a 63% reduction in motor and phonic symptoms since surgery. The patient’s average YGTSS score reduction 6 months to 37 months poststimulation was 65% (Figure 1B). However, the fluctuations in his Y-BOCS and YGTSS scores immediately after surgery and after DBS was turned on diminish our confidence in ascribing his symptom improvement completely to DBS.
The patient’s fluvoxamine dose has remained at 100 mg twice daily, while risperidone was decreased to 2 mg daily. His current DBS parameters are (right) 0+/1−, 6.5V (6.5 mA); 100 µsec; 130 Hz and (left) 0+/1−, 5V(5.2 mA); 100 µsec; 130 Hz.
As expected, the patient continued to experience functional impairment even as his OCD and depression improved. During the first year after surgery, he found few productive ways to fill the time that he previously spent performing rituals. He spent his time playing on his computer tablet and eating junk food, resulting in weight gain of 34 pounds.
About a year after DBS surgery, he participated for 6 months in a weekly social skills group for adults with ASD. Two years after stimulation was activated, he took additional steps toward finding a meaningful life. He was thrilled with new skills gained from taking math and computer classes, and he began volunteer work at a food bank. He found meaning in this work, and he reported feeling better about himself because he received positive feedback from his supervisor and peers. His weight has stabilized, and he now does his own banking online and is working toward living independently. The patient reports that he is much happier with his life.
Discussion
This case highlights several unique features related to the use of DBS in a patient with Tourettic OCD with comorbid MDD and ASD. When patients undergo DBS of the ventral capsule/ventral striatum for OCD, comorbid conditions such as depression may also improve (25, 26). However, other comorbid conditions, such as autism, should not be expected to improve, and those symptoms may come to the forefront as OCD symptoms recede. Clinicians need to vigilantly address comorbid conditions so that reductions in OCD symptoms may ultimately lead to improved overall quality of life. Initially, the above patient’s dramatic reduction in OCD symptoms led to some setbacks. However, with the support of his mother and treatment team, he has taken positive steps toward increasing his productivity and sense of purpose in life.
DBS for severe, treatment-refractory OCD (including Tourettic OCD) in patients who have received adequate pharmacotherapy and psychotherapy should be considered. In addition, use of the Y-BOCS and YGTSS should be considered in the assessment of patients with Tourettic OCD. Comorbid high-functioning ASD should not be an absolute contraindication to DBS. Families should be involved in the patient’s assessment and ongoing treatment, particularly when the patient seems to have limited self-awareness, considering information from both the patient and the family. Patients and their families should be educated about which symptoms DBS may help and which are not likely to improve. Furthermore, clinicians should be vigilant about treating comorbid conditions, which may become a more prominent foci of attention as OCD symptoms decrease. Patients and families should be informed that DBS is not a procedure that will fix OCD; rather, it is a procedure that may lead to symptom reduction when the patient participates actively in ongoing follow-up treatment, including continued engagement in exposure and response prevention and medication management.
1. : The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication . Mol Psychiatry 2010 ; 15 ( 1 ): 53 – 63 Crossref, Medline, Google Scholar
2. : The Brown Longitudinal Obsessive Compulsive Study: clinical features and symptoms of the sample at intake . J Clin Psychiatry 2006 ; 67 : 703 – 711 Crossref, Medline, Google Scholar
3. : Obsessive-compulsive and tic-related disorders . Child Adolesc Psychiatr Clin N Am 2012 ; 21 : 555 – 571 Crossref, Medline, Google Scholar
4. : Obsessive-compulsive disorder and autism spectrum disorders: longitudinal and offspring risk . PLoS One 2015 ; 10 : e0141703 Crossref, Medline, Google Scholar
5. : Obsessive-compulsive symptoms in parents of Tourette syndrome probands and autism spectrum disorder probands . Psychiatry Clin Neurosci 2004 ; 58 : 348 – 352 Crossref, Medline, Google Scholar
6 : Treatments for obsessive-compulsive disorder comorbid with autism spectrum disorder . Boston ,
7 : Treatment-refractory obsessive-compulsive disorder: methodological issues, operational definitions and therapeutic lines . Prog Neuropsychopharmacol Biol Psychiatry 2006 ; 30 : 400 – 412 Crossref, Medline, Google Scholar
8 : Response versus remission in obsessive-compulsive disorder . J Clin Psychiatry 2006 ; 67 : 269 – 276 Crossref, Medline, Google Scholar
9 : Deep brain stimulation for treatment-refractory obsessive compulsive disorder: a systematic review . BMC Psychiatry 2014 ; 14 : 214 Crossref, Medline, Google Scholar
10 : Deep brain stimulation for obsessive compulsive disorder: a review of results by anatomical target . Ment Illn 2018 ; 10 : 7900 Medline, Google Scholar
11. : Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders . J Neurol Neurosurg Psychiatry 2014 ; 85 : 1003 – 1008 Crossref, Medline, Google Scholar
12. : Nucleus accumbens deep brain stimulation for obsessive-compulsive disorder and aggression in an autistic patient: a case report and hypothesis of the role of nucleus accumbens in autism and comorbid symptoms . World Neurosurg 2019 ; 125 : 387 – 391 Crossref, Medline, Google Scholar
13. : Bilateral deep brain stimulation of the nucleus accumbens for comorbid obsessive compulsive disorder and Tourette’s syndrome . BMJ Case Rep 2012 ; 2012 : bcr2012006579 Crossref, Medline, Google Scholar
14. : Deep brain stimulation for the obsessive-compulsive and Tourette-like symptoms of Kleefstra syndrome . Neurosurg Focus 2015 ; 38 : E12 Crossref, Medline, Google Scholar
15. : Is deep brain stimulation effective and safe for patients with obsessive compulsive disorder and comorbid bipolar disorder? J Affect Disord 2020 ; 264 : 69 – 75 Crossref, Medline, Google Scholar
16. : Deep brain stimulation for Tourette’s syndrome . Transl Neurodegener 2020 ; 9 : 4 Crossref, Medline, Google Scholar
17. : Deep brain stimulation in the nucleus accumbens for intractable Tourette’s syndrome: follow-up report of 36 months . Biol Psychiatry 2009 ; 65 : e5 – e6 Crossref, Medline, Google Scholar
18. : Pallidal deep brain stimulation combined with capsulotomy for Tourette’s syndrome with psychiatric comorbidity . J Neurosurg 2019 ; 131 : 1788 – 1796 Crossref, Medline, Google Scholar
19. : Psychiatric classifications: validity and utility . World Psychiatry 2016 ; 15 : 26 – 31 Crossref, Medline, Google Scholar
20. : Autistic syndrome with onset at age 31 years: herpes encephalitis as a possible model for childhood autism . Dev Med Child Neurol 1991 ; 33 : 920 – 924 Crossref, Medline, Google Scholar
21. : Autism associated with anti-NMDAR encephalitis: glutamate-related therapy . Front Psychiatry 2019 ; 10 : 440 Crossref, Medline, Google Scholar
22. : Tic or compulsion? It’s Tourettic OCD . Behav Modif 2005 ; 29 : 784 – 799 Crossref, Medline, Google Scholar
23. : Microlesion effect as a predictor of the effectiveness of subthalamic deep brain stimulation for Parkinson’s disease . Stereotact Funct Neurosurg 2013 ; 91 : 12 – 17 Crossref, Medline, Google Scholar
24. : Managing patients with psychiatric conditions treated with deep brain stimulation ; in Deep Brain Stimulation Management , 2nd ed . Edited by Marks WJ , Cambridge, United Kingdom , Cambridge University Press , 2015 Crossref, Google Scholar
25. : Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience . Mol Psychiatry 2010 ; 15 : 64 – 79 Crossref, Medline, Google Scholar
26. : Deep brain stimulation for the treatment of addiction: basic and clinical studies and potential mechanisms of action . Psychopharmacology (Berl) 2013 ; 229 : 487 – 491 Crossref, Medline, Google Scholar



