The Diagnostic Challenge of Young-Onset Dementia Syndromes and Primary Psychiatric Diseases: Results From a Retrospective 20-Year Cross-Sectional Study
Abstract
Objective:
Distinguishing a dementia syndrome from a primary psychiatric disease in younger patients can be challenging and may lead to diagnostic change over time. The investigators aimed to examine diagnostic stability in a cohort of patients with younger-onset neurocognitive disorders.
Methods:
A retrospective review of records was conducted for patients who were admitted to an inpatient neuropsychiatry service unit between 2000 and 2019, who were followed up for at least 12 months, and who received a diagnosis of young-onset dementia at any time point. Initial diagnosis included Alzheimer’s disease-type dementia (N=30), frontotemporal dementia (FTD) syndromes (N=44), vascular dementia (N=7), mild cognitive impairment (N=10), primary psychiatric diseases (N=6), and other conditions, such as Lewy body dementia (N=30).
Results:
Among 127 patients, 49 (39%) had a change in their initial diagnoses during the follow-up period. Behavioral variant FTD (bvFTD) was the least stable diagnosis, followed by dementia not otherwise specified and mild cognitive impairment. Compared with patients with a stable diagnosis, those who changed exhibited a higher cognitive score at baseline, a longer follow-up period, greater delay to final diagnosis, and no family history of dementia. Patients whose diagnosis changed from a neurodegenerative to a psychiatric diagnosis were more likely to have a long psychiatric history, while those whose diagnosis changed from a psychiatric to a neurodegenerative one had a recent manifestation of psychiatric symptoms.
Conclusions:
Misdiagnosis of younger patients with neurocognitive disorders is not uncommon, especially in cases of bvFTD. Late-onset psychiatric symptoms may be the harbinger to a neurodegenerative disease. Close follow-up and monitoring of these patients are necessary.
Young-onset dementia, a dementia syndrome with onset under the age of 65, accounts for approximately 8% of all people with dementia (1). The presenting symptoms of young-onset dementia may be atypical and differ from those seen in late-onset dementia (2). The differential diagnosis of young-onset dementia can be broad (3) and includes neurological, neurodegenerative, and primary psychiatric disorders. The clinical distinction can be most difficult in patients with this type of dementia and prominent frontal or white matter change, as seen in the behavioral variant of frontotemporal dementia (bvFTD) (4, 5), the “frontal variant” of Alzheimer’s disease (6), white matter disorders, such as leukodystrophies (7, 8), or vascular dementias (9, 10). Such patients may commonly present with personality, mood, or behavioral changes, including apathy, social withdrawal, mood disturbance, stereotyped/compulsive behaviors, disinhibition, or social inappropriateness.
Distinguishing a primary psychiatric disorder from a frontotemporal syndrome can be challenging (11, 12), especially in patients with a long-standing history of schizophrenia or bipolar disorder, given the potential overlap at the level of cognition and/or psychiatric symptomatology (13). In a proportion of patients with young-onset dementia, the difficulty in diagnosis may lead to diagnostic change over time from a psychiatric to a neurodegenerative diagnosis or vice versa. While some studies (13, 14) have described patients with a psychiatric diagnosis subsequently diagnosed with an underlying neurodegenerative condition, few studies have specifically addressed the question of diagnostic stability or change in patients with younger-onset dementia.
In a recent study, Perry et al. (15) examined diagnostic stability across neurodegenerative diseases in patients seen on at least two occasions in a tertiary memory service, aiming to identify factors that could influence diagnostic change. The investigators reviewed clinical records of patients diagnosed with bvFTD, language variants FTD, Alzheimer’s disease, corticobasal syndrome, and progressive supranuclear palsy. Diagnostic change occurred frequently in patients with bvFTD (32%) and was more common in patients with possible bvFTD (70%) compared with probable bvFTD (15%). In 22% of bvFTD patients, the diagnosis changed to a nondementia diagnosis, such as multiple sclerosis, or a psychiatric diagnosis, such as bipolar disorder.
Krudop et al. (16) examined patients presenting to a specialized memory clinic who were initially diagnosed as having bvFTD. After a 2-year follow-up period, 49% of these patients underwent a change in diagnosis. Thirty-two percent were reclassified with a primary psychiatric diagnosis, most commonly mood disorders, while 17% received other neurologic diagnoses, most commonly neurodegenerative diagnoses.
Woolley et al. (17) examined rates and risk factors for prior psychiatric diagnoses of neurodegenerative diseases (including Alzheimer’s, bvFTD, and primary progressive aphasia, among others) and reported that 28% of participants were initially given a psychiatric diagnosis. Of the dementia diagnoses, bvFTD was significantly more often initially misdiagnosed as a primary psychiatric condition, with depression being the most common diagnosis.
While the available studies have shown that diagnostic change is bidirectional (i.e., diagnoses may change from neurodegenerative to psychiatric or vice versa), individual studies have been largely unidirectional in that they have either focused on diagnostic change in patients initially diagnosed with dementia (15, 16) or have looked retrospectively at rates of prior psychiatric diagnoses in patients with neurodegenerative diseases (17). In the present study, we aimed to address this limitation by examining diagnostic stability in both directions by investigating diagnostic change in a cohort of patients with younger-onset neurocognitive disorders referred to a neuropsychiatry service.
METHODS
Study Design and Participants
We undertook a retrospective, cross-sectional study of all patients who were admitted to the neuropsychiatry inpatient unit at Royal Melbourne Hospital between 2000 and 2019 for diagnostic work-up. All data were retrieved from electronic medical files, including discharge summaries and outpatient letters. An independent investigator (P.T.), uninvolved in the patients’ care, reviewed the files. Inclusion criteria for the study are described below.
An inpatient diagnostic admission to neuropsychiatry between 2000 and 2019
At least one inpatient or outpatient follow-up (≥12 months) assessment with neuropsychiatry
A diagnosis of younger-onset dementia or mild cognitive impairment at any time point between 2000 and 2019
The study was conducted in accordance with the Declaration of Helsinki and approved by the Melbourne Health Ethics Committee.
Demographic and Clinical Variables
Patients’ files were reviewed for the following information: age (at symptom onset and hospital admission), sex, education, alcohol and tobacco consumption, medical comorbidities, presenting symptoms, psychiatric history, and family history.
Diagnostic Information
Both inpatient and outpatient assessments included evaluation by a multidisciplinary team, consisting of a neuropsychiatrist, a neurologist, a neuropsychologist, an occupational therapist, and a social worker. Rating instruments included the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG) (18) and the Mini-Mental State Examination (19) to assess bedside cognition and the Global Assessment of Functioning scale to assess level of function. Investigations included blood and urine tests, structural and functional imaging of the brain (MRI, single-photon emission computed tomography, positron emission tomography [PET]), and cerebrospinal fluid (CSF) analysis. Genetic testing was performed where clinically indicated in the presence of a strong family history. Following all multidisciplinary and multimodal assessments, a consensus diagnosis was made for all patients and recorded in the clinical file. Each patient’s inpatient and outpatient medical records were reviewed to collate diagnoses made through the patient’s time with neuropsychiatry.
All diagnoses (initial and final) were made based on established contemporaneous diagnostic criteria (DSM-IV and DSM-5) (20–23).
Diagnoses were grouped into the following six categories: Alzheimer’s-type dementia, FTD (including bvFTD, semantic and nonfluent variant primary progressive aphasia, and FTD with motor neuron disease [FTD-MND]), vascular dementia (VaD), mild cognitive impairment, primary psychiatric diseases (including major depressive disorder, bipolar affective disorder, and schizophrenia), and other diseases not categorized in any of the previous groups, such as Lewy body dementias (including Parkinson’s disease dementia and dementia with Lewy bodies), progressive supranuclear palsy, corticobasal ganglionic degeneration, and dementia not otherwise specified.
Diagnostic Outcomes
Patients were categorized into four groups, as described below, depending on initial and final diagnosis.
No diagnostic change
Change from a neurodegenerative diagnosis to another neurodegenerative or neurological condition (e.g., from dementia not otherwise specified to Alzheimer’s disease)
Change from a neurodegenerative diagnosis to a primary psychiatric diagnosis (e.g., from bvFTD to schizophrenia)
Change from a psychiatric diagnosis to a neurodegenerative diagnosis (e.g., from major depressive disorder to bvFTD)
We considered patients whose diagnosis was switched from a neurodegenerative to a psychiatric disorder (or vice versa) as having a “between-category” diagnostic change, while change within the neurodegenerative category was considered a “within-category” change.
Statistical Analysis
Continuous variables are reported as means with standard deviations. Categorical variables are reported as frequencies (%). As a result of our small sample size and after testing for normality, Mann-Whitney U tests were performed to test differences in numerical variables between diagnostically stable and unstable groups. Pearson’s chi-square tests of independence were performed to compare dichotomous variables and determine differences in psychiatric and family history across the categories of change. A two-sided p value <0.05 was considered statistically significant. Statistical analysis was performed with SPSS, version 25 (IBM, Armonk, N.Y.).
RESULTS
Baseline Demographic and Clinical Characteristics
A total of 462 patients were identified as having a dementia syndrome or mild cognitive impairment. Of these, 195 individuals were excluded, either because they were not followed up (N=156) or were followed up for less than 12 months (N=39). We excluded patients whose age at symptom onset was >65 years (N=77). Patients diagnosed with genetically confirmed dementia disorders, such as Huntington’s disease and Niemann-Pick disease type C, at their first inpatient admission were not included in our analysis. A total of 127 patients met inclusion criteria and were included in the analysis (Figure 1).
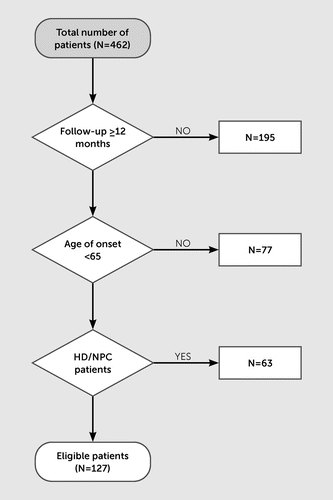
FIGURE 1. Eligibility criteria for study inclusion among patients admitted to an inpatient neuropsychiatry service unit between 2000 and 2019 and received a diagnosis of young-onset dementiaa
a HD=Huntington’s disease; NPC=Niemann-Pick disease type C.
Of the 127 patients who met inclusion criteria, 82 were male (64.6%), the mean age at onset was 50.1 years (SD=8.6), the mean duration of symptoms until initial diagnosis was 3.1 years (SD=2.2), and the mean time of follow-up was 40 months (SD=28.1). A detailed summary of participants’ baseline characteristics is presented in Table 1.
| Characteristic | No change (N=78) | Change (N=49) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age at onset (years) | 49.7 | 8.8 | 50.7 | 8.2 |
| Age at admission (years) | 52.4 | 9.4 | 53.7 | 8.4 |
| Time until initial diagnosis (years) | 3.2 | 2.1 | 3 | 2.1 |
| Months of follow-up | 35.3 | 25 | 47.5 | 31.2 |
| Overall delay to final diagnosis (years) | 3.1 | 2 | 5.7 | 3.3 |
| Neuropsychiatry Unit Cognitive Assessment Tool score | 65.4 | 18.2 | 74.9 | 12.5 |
| N | % | N | % | |
| Male | 48 | 61.5 | 34 | 69.4 |
| Hypertension | 12 | 15.4 | 15 | 30.6 |
| Diabetes mellitus | 6 | 7.7 | 9 | 18.4 |
| Dyslipidemia | 33 | 42.3 | 18 | 36.7 |
| Smoker | 18 | 23.1 | 13 | 26.5 |
| Alcohol consumption | 23 | 29.5 | 16 | 32.7 |
| Tertiary education | 24 | 32.0 | 15 | 32.6 |
| Family history of dementia | 37 | 48.1 | 10 | 20.8 |
TABLE 1. Baseline characteristics at first presentation among young-onset dementia patients who met study inclusion criteria
Patients who were not followed up by neuropsychiatry (N=156) included patients who returned to private or public specialist care or were lost to follow-up. Compared with the 127 patients who met inclusion criteria, these patients were older at admission (mean age: 57.6 years versus 51.0 years, p<0.05) and at symptom onset (mean age: 54.4 years versus 47.8 years, p<0.05). The most common diagnoses among patients who were not followed up were Alzheimer’s disease (21.9%) and VaD (12.4%).
Diagnostic Change
The key finding among patients who met inclusion criteria was that there was a change of diagnosis during the study period for 39% of these patients (N=49). bvFTD was the least stable diagnosis, with almost half of patients (47.1%) undergoing a diagnostic change. Dementia not otherwise specified was the second least stable diagnosis, with 16% of patients shifting to another neurodegenerative/neurological or psychiatric condition. Changes in Alzheimer’s and VaD dementia were less common (10.2% and 4.1%, respectively). No patients diagnosed with a language variant FTD (semantic dementia [N=6]), nonfluent primary progressive aphasia (N=3), or FTD-MND (N=1) had a diagnostic change. Male participants had a change more frequently than females (69.4% and 30.6%, respectively). Changes in the 49 patients presenting with unstable diagnoses are summarized in Table 2.
| Initial diagnoses | Final diagnoses | Total | |||||
|---|---|---|---|---|---|---|---|
| AD-type dementia | FTD | VaD/mixed AD-VaD | Mild cognitive impairment | Primary psychiatric disease | Other | ||
| AD-type dementia | 0 | 0 | 1 | 0 | 1 | 3 | 5 |
| FTD | 2 | 1b | 0 | 1 | 7 | 5 | 16 |
| VaD | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Mild cognitive impairment | 0 | 0 | 0 | 0 | 1 | 5 | 6 |
| Primary psychiatric disease | 0 | 3 | 0 | 0 | 0 | 3 | 6 |
| Other | 1 | 2 | 0 | 2 | 2 | 7 | 14 |
| Total | 4 | 6 | 1 | 3 | 12 | 23 | 49 |
TABLE 2. Changes between initial and final diagnoses during the follow-up period among patients with young-onset dementiaa
For the patients initially diagnosed with bvFTD, five later received “other” diagnoses, including hereditary spastic paraplegia, central nervous system (CNS) vasculitis, Parkinson’s disease dementia, and dementia not otherwise specified, while one patient within the FTD group was rediagnosed as having the semantic variant. Patients presenting with an unclear condition at first admission (categorized in the “other” group) had their diagnosis revised at subsequent visits to more specific diseases, such as multiple system atrophy, Parkinson’s disease dementia, paraneoplastic encephalitis, or mild cognitive impairment. Two patients diagnosed with progressive supranuclear palsy and corticobasal ganglionic degeneration at first admission were switched to bvFTD and dementia with Lewy bodies, respectively.
Comparison of Patients With No Change in Diagnosis With Those With a Diagnostic Change
Compared with patients with a stable diagnosis, patients with a diagnostic change exhibited a higher NUCOG score at baseline (mean=74.9, 95% CI=70.8, 79.1 versus mean=65.4, 95% CI=60.8, 69.9, p<0.05), a longer follow-up period (mean=47.5 months, 95% CI=38.5, 56.5 versus mean=35.3 months, 95% CI=29.7, 41, p<0.05), and a greater delay to final diagnosis (mean=5.7 years, 95% CI=4.7, 6.7 versus mean=3.1 years, 95% CI=2.6, 3.6, p<0.01) and were more likely to have a history of hypertension (30.6% versus 15.4%, χ2=4.1, p<0.05). Patients with a family history of dementia were more likely to have a stable diagnosis compared with those who did not report family history (48.1% versus 20.8%, χ2=9.3, p<0.01). No difference was detected between the two groups regarding age at onset, education, smoking habits, and alcohol consumption.
Categories of Diagnostic Change
Within-category diagnostic change.
Of the 49 patients whose initial diagnosis was revised, 30 (23.6%) underwent a within-category diagnostic change. For two patients who were initially diagnosed with bvFTD, the change to a diagnosis of Alzheimer’s disease was influenced by CSF and amyloid PET biomarkers. Another participant had the diagnosis revised from bvFTD to hereditary spastic paraplegia after undergoing genetic testing. Genetics led to a change from mild cognitive impairment to Huntington’s disease in one patient. Patients given a diagnosis of dementia not otherwise specified at the time of first admission had a more precise diagnosis of bvFTD, posterior cortical atrophy, and chronic inflammatory encephalopathy when they were subsequently seen at follow-up. Two patients with Alzheimer’s disease developed common vascular copathology, while three cases of mild cognitive impairment were revised to Parkinson’s disease dementia (N=2) and progressive supranuclear palsy (N=1).
For the 49 patients who had a diagnostic change during follow-up, those who reported a negative psychiatric history were more likely to have a within-category change than those who reported a positive psychiatric history (χ2=4.8, p<0.05; odds ratio=4.6, 95% CI=1.1, 19.4). No differences were detected in terms of medical comorbidities (hypertension, diabetes mellitus, and dyslipidemia) and alcohol and tobacco consumption.
Between-category diagnostic change.
Nineteen patients (14.9% of the total group [N=19/127], 39.8% of the change group [N=19/49]) exhibited a between-category diagnostic change and were further divided into two subtypes as described below.
Thirteen patients (10.2% of the total group) switched from a neurodegenerative to a psychiatric diagnosis (subtype I).
Six patients (4.7% of the total group) switched from a psychiatric to a neurodegenerative diagnosis (subtype II).
Further clinical details of these 19 patients are presented in Table 3. Of the 13 patients in the first subtype (patients 1–13; Table 3), six had schizophrenia, three had bipolar disorder, one had depression, and three had no evidence of a progressive neurodegenerative disorder. Of the six patients with the second subtype (patients 14–19; Table 3), half developed an FTD diagnosis.
| Patient | Age at symptom onset (years) | Sex | Clinical presentation | Neuroimaging results at first admission | Psychiatric history | Initial diagnosis | Final diagnosis | Reason for change | Months of follow-up | Number of visits |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 48 | Male | Fluctuating memory impairment, apathy, and irritability | No abnormalities detected | Nil | Fronto-striatal dementia NOS | No progressive neurological or neurodegenerative disorder | No evidence of progression | 24 | 2 |
| Patient 2 | 54 | Female | Cognitive impairment, psychotic symptoms, and agitation | Atrophy in frontal and temporal regions; moderate hypoperfusion bilaterally involving all lobes | Long-standing history of mood difficulties; 1-year prior experienced delirium and psychosis | AD | Schizoaffective disorder | Relative functional stability/no evidence of progressive dementia | 84 | 2 |
| Patient 3 | NR | Male | STM impairment and gait disturbance | Marked atrophy and moderately severe atrophy of frontal and temporal regions | Chronic history of treatment-refractory schizophrenia | bvFTD | Paranoid schizophrenia | Improvement of cognitive impairment/lack of progression | 50 | 3 |
| Patient 4 | 50 | Male | Disinhibition | Severe atrophy of frontotemporal lobes and hypoperfusion | BPAD in the past 8 years | bvFTD | BPAD | No significant progression on serial imaging >5 years | 115 | 13 |
| Patient 5 | 36 | Male | Functional decline and STM impairment | Bifrontal sulcal prominence and prefrontal, frontal, and anterior parietal cortical hypoperfusion | Chronic schizophrenia | bvFTD | Schizophrenia | Stability of cognition on serial neuropsychology reports and MRI scans | 58 | 11 |
| Patient 6 | 32 | Male | Inappropriate behavior, amotivation, and specific dietary preferences | Moderate generalized atrophy and mild hypoperfusion of anterior temporal poles | Schizophrenia from age 22 | bvFTD | Schizophrenia | Improvement of behavioral and cognitive problems | 42 | 8 |
| Patient 7 | 42 | Male | Poor concentration, parkinsonism | No abnormality detected | BPAD diagnosed at age 20 | bvFTD | BPAD | Lack of progressive changes on neuroimaging | 16 | 2 |
| Patient 8 | 50 | Female | Inattention, cognitive slowing, reduced problem solving, and parkinsonism | Mild hypoperfusion in the temporal and parietal lobe | Postnatal depression, OCD behaviors, and delusional disorder | bvFTD | No progressive dementing disorder | Improvement on cognitive testing; parkinsonism resolved following cessation of antipsychotics | 16 | 3 |
| Patient 9 | 53 | Female | Symptoms of anxiety and somatic complaints | Moderate volume loss and hypoperfusion of frontal and parietal lobes | Chronic schizophrenia | bvFTD | Schizophrenia | Stable imaging; improvement on neuropsychology assessments | 12 | 3 |
| Patient 10 | 51 | Male | STM impairment and inappropriate behavior | Mild atrophy and extensive hypoperfusion bilaterally in parietal, temporal, and frontal lobes | Nil | VaD | Factitious disorder | Inconsistency between functioning and reported level of disability | 47 | 2 |
| Patient 11 | 60 | Female | STM impairment and anxiety | Atrophy most prominent in parietal regions and hypoperfusion in parietal and temporal lobes | Ongoing symptoms of dysthymia and anxiety | Mild cognitive impairment due to cerebrovascular disease | Depression | Lack of progression | 17 | 4 |
| Patient 12 | 52 | Male | STM impairment, apathy, limited insight, and attentional decline | Global atrophy and frontotemporal hypoperfusion | BPAD from age 22 | Dementia NOS | BPAD | Presentation consistent with long-standing BPAD | 18 | 2 |
| Patient 13 | 44 | Male | STM and attentional problems | No marked abnormalities | Experienced depressive symptoms 3 years prior | Dementia NOS | Schizophrenia | Lack of deterioration | 48 | 3 |
| Patient 14 | 56 | Male | Disinhibition, aggressiveness | Mild hypoperfusion in left parietal, anteriortemporal, and cerebellar lobes | Nil | No diagnosis | Dementing disorder NOS | Slowly progressing illness; behavioral disturbance; significant impairments in executive function; and visuospatial abilities | 94 | 2 |
| Patient 15 | 38 | Male | Significant memory and concentration impairment | Temporal hypoperfusion | Depression diagnosed 3 years prior | Depressive episode in remission | Dementia NOS | Clinical presentation and imaging results consistent with a progressive condition | 60 | 3 |
| Patient 16 | 51 | Female | STM problems and social withdrawal | Reduction in volume of frontal and temporal lobes bilaterally | Experienced psychotic symptoms 4 years prior | Late-onset psychosis | bvFTD | Deterioration in mental state | 12 | 2 |
| Patient 17 | 48 | Male | Disinhibition, apathy, and poor self-care | Cerebral volume at thelower limit of age-appropriate range | Depression diagnosed 2 years prior | Depression | bvFTD | Presentation and imaging results strongly suggestive of bvFTD | 30 | 4 |
| Patient 18 | 59 | Female | Cognitive impairment and depressive symptoms | Caudate atrophy | Experienced depressive symptoms | Depression | HD | Genetics (43 CAG repeats in HTT gene) | 65 | 1 |
| Patient 19 | 58 | Female | Treatment-resistant depression | Mild hypometabolism offrontal and temporal lobes | Diagnosed with depression for the past 10 years | Depression | bvFTD | Deterioration in cognition and imaging results suggestive of bvFTD | 72 | 6 |
TABLE 3. Clinical details of patients with a between-category diagnostic changea
There was no difference between these two subtypes in the age at which participants first exhibited symptoms (subtype I: mean age=47.6 years versus subtype II: mean age=51.6 years, p=0.32). Of the participants in this between-category diagnostic change group, 84.2% (N=16) reported a positive history of psychiatric symptoms (depressive, psychotic). The presence of cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidemia, and smoking) was not associated with a diagnostic change in this group.
Patients in subtype I (i.e., those who changed from a neurodegenerative to a psychiatric diagnosis) were more likely to have a long psychiatric history (>10 years, N=8/13; 61.5%) compared with patients in subtype II (i.e., those who switched from a psychiatric to a neurodegenerative diagnosis), the majority of which had a recent manifestation of psychiatric symptoms (<10 years, N=5/6; 83.3%) (χ2=7.2, p<0.05).
DISCUSSION
This study examined bidirectional diagnostic stability across neurodegenerative and psychiatric diseases in a cohort of patients diagnosed with younger-onset dementia at presentation or subsequent follow-up in a single tertiary specialist clinical service. Several important findings emerged from this investigation. Of 127 patients, 49 (39%) had their initial diagnoses revised during the follow-up period. The observed diagnostic changes included change from neurodegenerative disease to another neurodegenerative or neurological condition (24%), from a neurodegenerative diagnosis to a psychiatric diagnosis (10%), and from a psychiatric diagnosis to a neurodegenerative diagnosis (5%). Alzheimer’s disease-type dementia was the most stable diagnosis over time. Consistent with previous literature, a diagnosis of bvFTD was found to be the most likely diagnosis to change during the follow-up period. For patients with a diagnosis of bvFTD, their diagnosis changed to another neurodegenerative or neurological diagnosis (24%) or to a psychiatric diagnosis (21%). On the other hand, 60% of patients finally diagnosed with bvFTD were given an initial diagnosis of depression, a finding consistent with that of Woolley et al. (17). Finally, we identified that a higher score on cognitive testing at baseline, a negative family history of dementia, and a positive psychiatric history were associated with a greater likelihood of diagnostic change.
From a clinical and patient perspective, the most significant diagnostic changes were the between-category changes (i.e., when the diagnosis changed from neurodegenerative to psychiatric or vice versa). In 19 patients who presented with a between-category change, the presence and duration of psychiatric symptoms played a significant role in the reconsideration of the patients’ initial diagnosis. Eight of the nineteen patients (42%) had a psychiatric history >10 years at the time of an initial diagnosis of a dementia. In all eight patients, the initial dementia diagnosis was subsequently revised to a psychiatric diagnosis based on lack of progression and, in some cases, improvement of cognitive impairment. Eight of the 19 patients (42%) had a history of psychiatric symptoms <10 years. More than half of these eight patients, who were initially diagnosed with a psychiatric condition (63%), were subsequently diagnosed with a neurodegenerative disease. It was believed that in these individuals, the late-onset psychiatric symptoms reflected a prodromal phase of an underlying neurodegenerative condition.
For the patients with a within-category change (N=30), the bvFTD diagnosis was the most frequent to change to another neurodegenerative or neurological condition (30%, N=9). These final diagnoses had a wide range, including spastic paraplegia, CNS vasculitis, Parkinson’s disease dementia/VaD, Alzheimer’s disease, and semantic dementia, among others. Patients initially diagnosed with dementia not otherwise specified and mild cognitive impairment were given a more specific diagnosis over time, meaning that a longer follow-up period was required in these patients. Genetic testing also influenced diagnostic change and should be taken into consideration in the presence of a strong family history and for further counseling.
The relationship between psychiatric diagnoses and younger-onset neurodegenerative dementia diagnoses has been investigated in several studies. Primary psychiatric diseases, such as major depressive disorder, schizophrenia, and bipolar disorder, are risk factors for the development of dementia later in life (24–28), while other studies have suggested that late-onset depression may represent a prodromal phase of dementia (29–31). Patients with a late-life onset of psychiatric manifestations or patients with a long psychiatric history presenting with cognitive decline should be carefully evaluated and followed up over time, as their symptomatology may signify an early phase of a neurodegenerative disease. In our cohort, patients who changed diagnosis were followed up significantly longer and had a greater delay to final diagnosis than those who remained stable.
Our findings should be considered “red flags,” as clinicians need to be careful and vigilant when evaluating and diagnosing patients with younger-onset dementia syndromes, especially in bvFTD and in treatment-resistant psychiatric symptoms. A validated checklist developed by Ducharme et al. (32) is a promising clinical tool aimed to improve diagnostic accuracy in these patients. Although clinical diagnostic criteria are available for the commonest disorders (Alzheimer’s disease, FTD, vascular dementia, and Lewy body dementia), they have only moderate sensitivity and specificity. Neurodegenerative diseases progress over time, and disease-modifying therapies are yet to be found. Such a diagnosis has a major impact on an adult’s life, especially in younger patients who are still likely to be active members of society with professional, financial, and caring responsibilities. On the other hand, psychiatric diseases can often be effectively treated, leading to improvement in symptoms and quality of life.
Limitations
The small number of patients included in our analysis and the small sample size of patients with a between-category diagnostic change limit the generalizability of our findings to the wider population of patients with younger-onset dementia or primary psychiatric diseases. A further limitation, related to the clinical basis of this study, is that the follow-up duration and intervals were not the same for all patients. We found that patients with a diagnostic change were followed up over a significantly longer period of time than those without a diagnostic change. The longer follow-up period may have increased the possibility of reconsideration and revision of the diagnosis.
Finally, our tertiary neuropsychiatric service generally sees patients with a complex constellation of cognitive, psychiatric, and neurological symptoms referred by other specialist physicians. We cannot comment on diagnostic change in those patients not referred to our service, but it is likely that patients referred to our service represent a more complex group diagnostically and are more likely to exhibit diagnostic uncertainty and change over time.
CONCLUSIONS
There can be significant overlap of psychiatric, neurological, cognitive, and even neuroimaging changes in younger-onset dementia syndromes and primary psychiatric diseases, causing diagnostic uncertainty and delay. Misdiagnosis is frequent, especially in cases of bvFTD. Because most primary psychiatric diseases occur in adolescence or early adulthood, the manifestation of late-onset (after 40 years old) psychiatric symptoms may be the harbinger to a neurodegenerative disease. On the other hand, a chronic mental illness may predispose to a dementing syndrome. Establishing an accurate diagnosis as early as possible is of paramount importance, because initiation of the appropriate therapeutic intervention may have its greatest potential in the early stages of a disease. Close monitoring and follow-up of these patients are required.
1 : Young onset dementia. Intern Med J 2016; 46:779–786Crossref, Medline, Google Scholar
2 : The diagnosis of young-onset dementia. Lancet Neurol 2010; 9:793–806Crossref, Medline, Google Scholar
3 : Young-onset dementia. Semin Neurol 2013; 33:365–385Crossref, Medline, Google Scholar
4 : Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol 2011; 10:162–172Crossref, Medline, Google Scholar
5 : The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry. J Neurol Neurosurg Psychiatry 2016; 87:501–511Crossref, Medline, Google Scholar
6 : The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain 2015; 138:2732–2749Crossref, Medline, Google Scholar
7 : The neuropsychiatry of adult-onset adrenoleukodystrophy. J Neuropsychiatry Clin Neurosci 1999; 11:315–327Link, Google Scholar
8 : Adrenoleukodystrophy: frequency of presentation as a psychiatric disorder. Biol Psychiatry 1987; 22:1375–1387Crossref, Medline, Google Scholar
9 : Behavioral differences between white matter lacunar dementia and Alzheimer’s disease: a comparison on the neuropsychiatric inventory. Dement Geriatr Cogn Disord 2000; 11:294–298Crossref, Medline, Google Scholar
10 : Frontal lobe dementia and subcortical vascular dementia: a neuropsychological comparison. Psychol Rep 2005; 96:141–151Crossref, Medline, Google Scholar
11 : Crossing borders between frontotemporal dementia and psychiatric disorders: an updated overview. J Alzheimers Dis 2020; 75:661–673Crossref, Medline, Google Scholar
12 : Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain 2020; 143:1632–1650Crossref, Medline, Google Scholar
13 : Frontotemporal dementia presenting as schizophrenia-like psychosis in young people: clinicopathological series and review of cases. Br J Psychiatry 2009; 194:298–305Crossref, Medline, Google Scholar
14 : Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain 2012; 135:693–708Crossref, Medline, Google Scholar
15 : Factors that predict diagnostic stability in neurodegenerative dementia. J Neurol 2019; 266:1998–2009Crossref, Medline, Google Scholar
16 : The pitfall of behavioral variant frontotemporal dementia mimics despite multidisciplinary application of the FTDC criteria. J Alzheimers Dis 2017; 60:959–975Crossref, Medline, Google Scholar
17 : The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry 2011; 72:126–133Crossref, Medline, Google Scholar
18 : The NUCOG: validity and reliability of a brief cognitive screening tool in neuropsychiatric patients. Aust N Z J Psychiatry 2006; 40:995–1002Crossref, Medline, Google Scholar
19 : “Mini-Mental State”: practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
20 : The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:263–269Crossref, Medline, Google Scholar
21 : Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
22 : Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134:2456–2477Crossref, Medline, Google Scholar
23 :
24 : Affective disorders and risk of developing dementia: systematic review. Br J Psychiatry 2013; 202:177–186Crossref, Medline, Google Scholar
25 : Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology 2010; 75:27–34Crossref, Medline, Google Scholar
26 : History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology 2008; 70:1258–1264Crossref, Medline, Google Scholar
27 : Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 2006; 63:530–538Crossref, Medline, Google Scholar
28 : Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol 2003; 60:753–759Crossref, Medline, Google Scholar
29 : 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry 2016; 3:628–635Crossref, Medline, Google Scholar
30 : Temporal relationship between depression and dementia: findings from a large community-based 15-year follow-up study. Arch Gen Psychiatry 2011; 68:970–977Crossref, Medline, Google Scholar
31 : Depression as a risk factor or prodromal feature for dementia? findings in a population-based sample of Swedish twins. Psychol Aging 2009; 24:373–384Crossref, Medline, Google Scholar
32 : The Frontotemporal Dementia versus Primary Psychiatric Disorder (FTD versus PPD) checklist: a bedside clinical tool to identify behavioral variant FTD in patients with late-onset behavioral changes. J Alzheimers Dis 2019; 67:113–124Crossref, Medline, Google Scholar



