Extracting Apathy From Depression Syndrome in Traumatic Brain Injury by Using a Clustering Method
Abstract
Objective:
Depression and apathy are common after traumatic brain injury (TBI), and different intervention strategies are recommended for each. However, a differential diagnosis can be difficult in clinical settings, especially given that apathy is considered to be a symptom of depression. In this study, the investigators aimed to isolate apathy from depression among patients with TBI and to examine whether apathy is exclusively associated with the amount of daily activity, as previously reported in the literature.
Methods:
Eighty-eight patients with chronic TBI completed the Japanese versions of the 21-item Beck Depression Inventory-II (BDI-II) and the Starkstein Apathy Scale (AS). Daily activity was measured with a 24-hour life log. A hierarchical cluster analysis was applied to divide the BDI-II data into separable components, and components’ correlations with results of the AS and 24-hour life log scale were evaluated.
Results:
The BDI-II and AS revealed that 37 patients (42.0%) had both depression and apathy. BDI-II data were classified into four separate clusters (somatic symptoms, loss of self-worth, affective symptoms, and apathy symptoms). Loss of self-worth and apathy symptoms subscores were significantly positively correlated with total AS score (r=0.32, p=0.002, and r=0.52, p<0.001, respectively). The apathy symptoms subscore was significantly correlated with the amount of daily activity (r=−0.29, p=0.009).
Conclusions:
The findings suggest that the BDI-II can differentiate between apathy and depression among patients with TBI, which is essential when selecting intervention options. Moreover, apathy symptoms predicted patients’ real-life daily activity.
Both depression and apathy are common psychiatric manifestations in various neurological and neuropsychiatric disorders, including Parkinson’s disease, dementia, schizophrenia, and traumatic brain injury (TBI) (1–7). However, depression and apathy are often mistaken for each other in both clinical and research settings. This confusion primarily stems from the definition of depression itself. The DSM-5, a mainstream psychiatric diagnostic system, defines depression as “depressed mood and/or a markedly diminished interest or pleasure” (8). As this definition indicates, depression is a complex of heterogeneous symptoms. In contrast, apathy, defined as a lack of motivation (9), is a narrow concept that refers to specific symptoms. As a result, apathy is included in the definition of depression as a partial constituent. Nonetheless, in neuropsychiatry, some authors have emphasized that apathy should be considered separately from depression (10). This view has been supported by the fact that these disorders respond to different interventions, including pharmacotherapy (7, 11, 12).
Several studies have examined this issue among patients with neurological and psychiatric disorders (13–18) and have successfully demarcated apathy from depression among patients with unipolar and bipolar depression (13), geriatric depression (18), and Alzheimer’s disease (16). Although patients with TBI have a high prevalence of both depression and apathy (19, 20), few studies have examined this issue. TBI is a serious public health problem that causes a variety of neurological and neuropsychiatric sequelae. Depression and apathy can lead to poor outcomes because of difficulties with rehabilitation or social integration and loss of vocational opportunities (21, 22). However, among patients with TBI, psychiatric sequelae vary depending on the severity of and time since injury (23, 24). Moreover, in some cases, the sequelae of psychiatric symptoms are difficult to assess because of patients’ lack of awareness (25). The few studies that have examined this issue among patients with TBI have found that the somatic component of depression can be distinguished from the other components of depression, although apathy has not been reported as an independent component (26, 27).
In the present study, we aimed to investigate the possibility of extracting the component of apathy from depression syndrome among patients with TBI. First, a cluster analysis was applied to data collected by using the 21-item Beck Depression Inventory–II (BDI-II) to examine whether the apathy component of depression is discriminable from its other components. Second, the validity of the extracted putative apathy subcomponent was examined. To do this, the correlation of this subcomponent score with a gold-standard apathy score, namely, the Starkstein Apathy Scale (AS) score, was determined. Finally, to further validate the significance of this subcomponent, the relationship between the apathy subcomponent score and daily activity data collected by using a self-monitoring life log was assessed.
Methods
Participants
Participants were recruited from the outpatient clinic of the neuropsychology unit at the Department of Psychiatry and from the Department of Neurosurgery, Kyoto University Hospital. Participants were diagnosed at the acute stage by neuroimaging, including brain computerized tomography (CT) or MRI, regardless of disorder of consciousness. Inclusion criteria for patients were as follows: presence of brain injury sustained through significant trauma; brain CT or MRI scans showing a specific lesion or possible diffuse pathology; older than age 18; injury occurring at least 3 months before the study; and the ability to provide informed consent to participate in the research. The exclusion criteria were as follows: history of another TBI with an altered state of consciousness, drug abuse, or neurological or psychiatric disorders before TBI onset.
TBI severity was defined according to the Glasgow Coma Scale (GCS) (28, 29) or the Japan Coma Scale (JCS) (30) score on hospital arrival. The JCS is a measure of the severity of impaired consciousness used in Japan. A significant relationship between JCS score and GCS score has already been reported (31), whereby the JCS determines injury severity as well as the GCS. To clarify patients’ characteristics, classification of TBI severity was also conducted on the basis of the duration of posttraumatic amnesia.
Regarding lesion location, frontal injury was defined as large focal lesions (e.g., lobar contusions, hematoma or hemorrhage >2 mm in diameter) or possible diffuse pathology (hemorrhage ≤2 mm in diameter) in prefrontal regions, according to radiological criteria. Previous studies have suggested that prefrontal regions underlie depression and apathy (24, 32). The radiological criteria for defining lesion location were as follows: for MRI, fluid attenuation inversion recovery, TR=4,200 ms, TE=94 ms, resolution 0.7 × 0.7 × 3.0 mm3, and for susceptibility weighted imaging, TR=28 ms, TE=20 ms, resolution 0.5 × 0.5 × 1.2 mm3. Of the 88 patients, five were unable to undergo MRI because of contraindications, and region location was instead defined with CT; these patients had gross lesions that were sufficiently detectable by CT. Thirty-five of the 88 patients were taking antipsychotic medication (antidepressants for depressive symptoms, pseudobulbar affect, and pain [N=24]; dopamine agonists for apathy symptoms [N=9]; antipsychotics for other psychiatric symptoms [N=2]).
This study was approved by the Committee on Medical Ethics of Kyoto University and was carried out in accordance with the Code of Ethics of the World Medical Association. Written informed consent was obtained after participants had been given a complete description of the study.
Questionnaires
BDI-II.
The Japanese version of the BDI-II was used to assess depressive symptoms (33). The self-rated BDI-II is the most commonly used scale for assessing depressive mood (33). Its 21 items are scored on a scale ranging from 0 to 3, and the total BDI score ranges from 0 to 64, with higher scores indicating more severe depressive mood.
AS.
The Japanese version of the AS was used to assess apathy (34, 35). The AS is a self-report questionnaire in which apathy symptoms are assessed by having patients answer 14 questions on a scale ranging from 0 to 3, with a maximum total score of 42. The criterion for a diagnosis of apathy is a score of 16 or more (36). Several instruments are used to measure apathy symptoms among patients with neurological and psychiatric disorders. The AS was selected because it has a Japanese version and is widely used in the evaluation of stroke patients.
To make clear the clinical characteristics of the patients in this study, patients’ classification using the cut-off values for the BDI-II and AS is presented in Figure 1A.
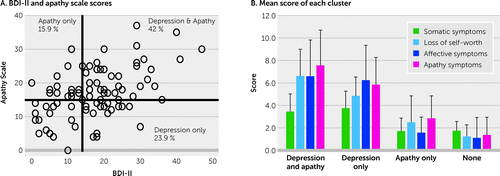
FIGURE 1. Scatter plot of the Beck Depression Inventory–II (BDI-II) and Apathy Scale (AS) scores among patients with traumatic brain injurya
a The x-axis in panel A shows the BDI-II score, and the y-axis shows the AS score. Patients who scored higher than 14 on the BDI-II were classified as having mild or more severe depressive symptoms. Patients who scored higher than the cut-off score of 16 on the AS were diagnosed as having apathy. According to these criteria, 42% of patients presented with both depression and apathy, 23.9% presented with depression alone without apathy, and 15.9% presented with apathy alone without depression. Panel B shows the depressive symptoms measured by BDI-II scores were divided into four separate clusters: somatic symptoms, loss of self-worth, affective symptoms, and apathy symptoms. According to the BDI-II and AS scores, patients were grouped for depression and apathy (BDI-II score ≥14 and AS score ≥16), depression only (BDI-II≥14), apathy only (AS≥16), and none.
24-hour life log and procedure.
To measure the amount of daily activity, a self-reported 24-hour life log questionnaire was used to document an entire day’s activities (37). The 24-hour life log is a part of questionnaire A from the survey administered every 5 years by the Statistics Bureau of the Ministry of Internal Affairs and Communications (http://www.stat.go.jp/english/data/shakai/index.htm). The questionnaire consists of a recording sheet of 24 hours divided into 15-minute blocks; for each block, participants are required to select their typical activity type from a list of 20 activities. The 20 activity items were categorized into three domains: primary activities (i.e., eating meals, commuting to work or school, job work performance, school work performance, domestic work, caring or nursing, child care, and shopping); secondary activities (i.e., noncommute travel; television, radio, newspaper, or magazine consumption; learning or self-development; hobbies and amusements; sports; volunteer and community activities; other social activities; hospital visit or treatment; and other activities); and sleep, rest, and relaxation. These logs were used to calculate the daily time for and weekly range of each activity, in accordance with a previous study conducted by the authors (37). For each patient, the total number of hours of primary and secondary activities per week was determined using the recorded daily activities. More detailed information on the 24-hour life log is provided in the previous study (37).
Data Analyses
Clustering method.
To extract the component of apathy from depression syndrome, agglomerative hierarchical clustering analysis was applied. First, Spearman’s correlation coefficient between each of the 21 items of the BDI-II data from patients with TBI (N=88) was calculated. The resulting correlation coefficients were input into the clustering analysis. Initially, each data point (each item of the BDI-II) was considered an individual cluster. At each iteration, on the basis of their correlation coefficient, similar clusters were merged until the optimal number of clusters was reached. Finally, all clusters were merged and visualized using a dendrogram. On the basis of the dendrograms, the 21 BDI-II items were divided into clusters that align with clinical perspectives.
Multidimensional scaling.
On the basis of the Spearman’s correlation coefficients between the 21 BDI-II items, the representative distance between each BDI-II item was visualized using multidimensional scaling, which is a technique for visualizing the level of similarity between items. To visualize the relationship between each item of the BDI-II and the AS score, the total AS score was included in calculations of the correlation coefficient.
Statistical approach.
To examine the validity of the extracted putative apathy subcomponent derived from the BDI-II, Spearman’s correlation analyses were performed between the subscore of each cluster from the BDI-II and the AS score.
Moreover, to validate the significance of the apathy subcomponent derived from the BDI-II, correlation analyses were performed among depression, apathy, and amount of daily activity recorded on the 24-hour life log. First, Spearman’s correlation coefficient was calculated between the total scores of the BDI-II, AS, and amount of daily activity. Second, correlation analyses between the subscore of each BDI-II cluster and the amount of daily activity were performed.
All analyses were conducted using R version 3.0.2 (R Foundation for Statistical Computing Platform). A p<0.05 with multiple comparisons (familywise error corrected) was considered statistically significant.
Results
Eighty-eight patients with TBI (64 men; mean age=41.9 years [SD=14.7]) participated in the study. Patients’ demographic and clinical characteristics are presented in Table 1.
| TBI | |||||
|---|---|---|---|---|---|
| Characteristics | Mean | SD | N | % | Range |
| Age (years) | 41.9 | 14.7 | — | — | 19–70 |
| Male | — | — | 64 | 72.7 | — |
| Right handedness | — | — | 83 | 94.3 | — |
| Injury type | |||||
| Motor vehicle accident | — | — | 76 | 86.4 | — |
| Fall | — | — | 9 | 10.2 | — |
| Other | — | — | 3 | 3.4 | — |
| Time from injury (months) | 97.4 | 104.9 | — | — | 3–418 |
| Duration of posttraumatic amnesia (days)b | 48.8 | 56.3 | — | — | 0–300 |
| Severity of TBIc | — | — | — | — | — |
| Severe | — | 58 | 65.9 | ||
| Moderate | — | — | 12 | 13.6 | — |
| Mild | — | — | 18 | 20.5 | — |
| Lesion location, frontal injury | — | — | 69 | 78.4 | — |
TABLE 1. Demographic and clinical characteristics of patients with traumatic brain injury (TBI) (N=88)a
Prevalence of Depression and Apathy
Thirty patients (34.1%) had a BDI-II score lower than 13, which indicates extremely mild symptoms of depression. Twenty-three patients (26.1%) showed mild symptoms of depression (BDI-II score 14–19), 21 (23.9%) had moderate symptoms of depression (BDI-II score 20–28), and 14 (15.9%) had severe symptoms of depression (BDI-II score >29). In total, 58 patients (65.9%) who had a BDI-II score of more than 14 were classified as depressed, according to Beck’s criterion and a previous TBI study (38, 39). Fifty-one patients (57.9%) met the AS criteria for a diagnosis of apathy (an AS score of >16). Twenty-one (23.9%) patients met the BDI-II criteria for depression alone without concomitant apathy, 14 (15.9%) met the AS criteria for apathy alone and were not depressed, and 37 (42.0%) met the criteria for both depression and apathy (Figure 1A). Participants’ demographic and clinical characteristics by symptom category (depression only, apathy only, depression plus apathy, and no symptoms) are summarized in Table 2.
| Symptom category | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Depression plus apathy (N=37) | Depression only (N=21) | Apathy only (N=14) | None (N=16) | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 41.2 | 14.6 | 44.1 | 12.6 | 41.6 | 15.3 | 40.8 | 16.1 |
| Time from injury (months) | 95.6 | 104.7 | 122.1 | 110.8 | 79.4 | 83.7 | 84.4 | 104.9 |
| N | % | N | % | N | % | N | % | |
| Male | 26 | 70.3 | 15 | 71.4 | 10 | 71.4 | 13 | 81.3 |
| Severity (severe)a | 24 | 64.9 | 13 | 61.9 | 8 | 57.1 | 13 | 81.3 |
| Lesion (frontal injury) | 32 | 86.5 | 14 | 66.7 | 9 | 64.3 | 14 | 87.5 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Beck Depression Inventory-II total score | 24.2 | 7.8 | 20.7 | 5.3 | 8.6 | 3.5 | 5.5 | 3.7 |
| Somatic symptoms | 3.5 | 1.6 | 3.8 | 1.5 | 1.7 | 1.2 | 1.8 | 0.8 |
| Loss of self-worth | 6.6 | 2.4 | 4.9 | 1.7 | 2.5 | 2.4 | 1.3 | 1.0 |
| Affective symptoms | 6.6 | 3.2 | 6.2 | 3.1 | 1.6 | 1.4 | 1.1 | 1.8 |
| Apathy symptoms | 7.6 | 3.1 | 5.9 | 2.4 | 2.9 | 2.0 | 1.4 | 1.6 |
| Apathy Scale | 22.5 | 5.2 | 11.7 | 3.5 | 19.3 | 3.8 | 8.9 | 4.0 |
| Amount of activity (hours per week) | 89.6 | 19.2 | 98.3 | 13.1 | 96.6 | 15.8 | 102.9 | 12.8 |
| Primary activityb | 56.9 | 22.8 | 61.1 | 24.6 | 54.9 | 23.1 | 58.9 | 26.2 |
| Secondary activityc | 32.9 | 24.6 | 37.2 | 26.2 | 41.7 | 22.5 | 44.0 | 30.5 |
TABLE 2. Demographic and clinical characteristics of study participants by symptom category among patients with traumatic brain injury (N=88)
24-Hour Life Log Data
Eighty patients completed the 24-hour life log. The mean daily activity time (total duration of primary activities [e.g., work or school and domestic work] and secondary activities [e.g., hobbies and amusements, sports, and volunteer and community activities]) was 95.2 hours per week (SD=17.3). As a reference, the mean daily activity time for healthy adults is 102.9 hours per week (SD=12.7) (37). Compared with healthy adults, the amount of daily activity among patients with TBI was shorter by about 1 hour per day. The amount of activity in each group (depression plus apathy, depression only, apathy only, and no symptoms) is summarized in Table 2.
As a supplementary analysis, the amount of activity of patients who were or were not taking medication was compared to investigate the contribution of medication to amount of activity. Of these 80 patients, 21 were taking antidepressants; however, no significant difference was observed in their amount of activity compared with patients who were not taking medication (92.7±14.9 and 96.1±17.9 hours per week, respectively; t=−0.77, df=78, p=0.44).
Components by Hierarchical Clustering
Figure 2A shows a dendrogram used to visualize clusters calculated by correlation coefficients of the BDI-II items. A total of four clusters were found to categorize symptoms reported in the BDI-II. The first cluster included four symptoms: changes in appetite, changes in sleeping pattern, suicidal thoughts or wishes, and loss of interest in sex. This cluster was termed somatic symptoms because it included symptoms related to changes in physical conditions; however, the item “suicidal thoughts or wishes” does not fit the concept of somatic symptoms. The second cluster included five symptoms: pessimism, past failure, worthlessness, self-dislike, and guilty feelings. This cluster was termed loss of self-worth because it included symptoms related to self-worth. The third cluster included six symptoms: irritability, crying, punishment feelings, self-criticalness, sadness, and agitation. This cluster was termed affective symptoms because the majority of items in this cluster related to affective changes, although the items “punishment feelings” and “self-criticalness” might conceptually be more closely related to loss of self-worth. The fourth cluster also included six symptoms: concentration difficulty, tiredness or fatigue, loss of pleasure, indecisiveness, loss of energy, and loss of interest. This cluster was termed apathy symptoms because it mainly included motivational symptoms, although it also included other items such as “concentration difficulty” and “indecisiveness.” The percentage of cluster subscores of the mean total BDI-II score for all participants with TBI was 17% for somatic symptoms, 26% for loss of self-worth, 27% for affective symptoms, and 30% for apathy symptoms. The mean subscores of each cluster in each patient group (i.e., depression plus apathy, depression only, apathy only, and no symptoms) are shown in Table 2 and Figure 1B.
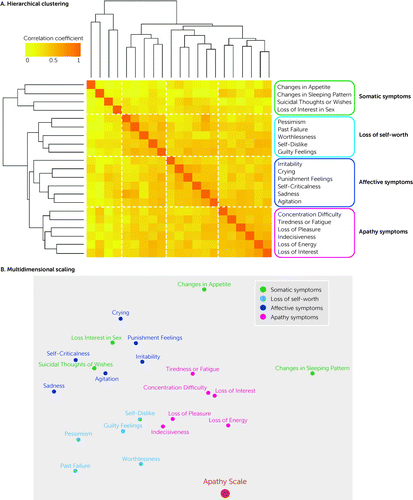
FIGURE 2. Hierarchical clustering and multidimensional scaling of Beck Depression Inventory–II (BDI-II) items among patients with traumatic brain injurya
aPanel A shows the dendrogram visualizing the four clusters of the BDI-II based on correlation coefficients between the 21 items of the BDI-II. The first cluster included four symptoms and was termed somatic symptoms. The second cluster included five symptoms and was termed loss of self-worth. The third cluster included six symptoms and was termed affective symptoms. The fourth cluster included six symptoms and was termed apathy symptoms. Darker boxes in this matrix indicate a more robust, higher correlation coefficient between the variables. Panel B shows a representation of the distances between each BDI-II item and the total Apathy Scale (AS) score. BDI-II items that were more similar are closer together in this figure than items that were less similar. Somatic symptoms, loss of self-worth, affective symptoms, and apathy symptoms were the clusters identified using hierarchical clustering. Each item in the apathy symptoms cluster and loss of self-worth clusters divided by the hierarchical clustering was also agglomerated on the plot by multidimensional scaling. Moreover, these clusters were adjacent to each other. The AS scores were close to the apathy symptoms cluster and loss of self-worth cluster that were divided by the hierarchical clustering.
Multidimensional Scaling
Multidimensional scaling representing the distances between each BDI-II item, and based on the correlation coefficients, is shown in Figure 2B.
Relationship Among BDI-II Score, AS Score, and Daily Activity
A positive correlation was found between the total BDI-II and AS scores (r=0.41, p<0.001). A significant negative correlation was found between total AS score and the amount of daily activity (r=−0.38, p<0.001). No significant correlation was found between total BDI-II score and daily activity (r=−0.21, p=0.06).
Relationship Between Each BDI-II Cluster and AS Score
The loss of self-worth subscore and apathy symptoms subscore were significantly positively correlated with total AS score (r=0.32, p=0.002, and r=0.52, p<0.001, respectively). No significant correlations were found between somatic symptoms and affective symptoms subscores and total AS score (Figure 3A).
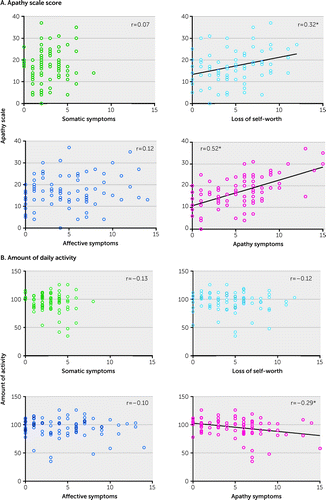
FIGURE 3. Correlation between score of each Beck Depression Inventory–II (BDI-II) cluster and the Apathy Scale (AS) score and amount of daily activitya
aPanel A shows significant positive correlations between the loss of self-worth, apathy symptoms, and total score of the AS. *p<0.05, Spearman’s correlation analyses (familywise error corrected). Panel B shows a significant negative correlation between the apathy symptoms subscore and amount of daily activity. *p<0.05, Spearman’s correlation analyses.
Relationship Between Each BDI-II Cluster and Daily Activity
A significant negative correlation was found between the apathy symptoms subscore and the amount of daily activity (r=−0.29, p=0.009). No significant correlations were found between the amount of daily activity and the somatic symptoms, loss of self-worth, and affective symptoms subscores (Figure 3B).
Discussion
The primary aim of this study was to determine whether apathy can be distinguished from the other components of depression among patients with TBI by using a common measure of depression. Nearly half of the patients had symptoms of both depression and apathy. The hierarchical clustering revealed that depressive symptoms measured by the BDI-II could be divided into four separate clusters (somatic symptoms, loss of self-worth, affective symptoms, and apathy symptoms). Among these clusters, the apathy symptom subscore showed a robust correlation with AS score. In addition, the apathy symptoms subscore was significantly correlated with the amount of daily activity, whereas no such relationship was seen with the subscores of the other clusters. Although many patients with TBI have symptoms of both depression and apathy, these results suggest that the apathy component is separable from broader depressive symptoms.
The BDI-II results revealed that 65.9% of patients with TBI in the present study had mild to severe depression. In previous studies, the reported prevalence of depression has been variable, ranging from 6% to 77% (19, 21, 40). The relatively higher prevalence reported in this study might be due to use of the BDI-II to diagnose depression rather than semistructured interviews of the DSM. However, it is also likely due to the characteristics of this study’s participants, who were recruited from the outpatient clinic of a university psychiatry department, which inevitably led to a higher percentage of patients with psychiatric symptoms. As for apathy, previous studies have reported a prevalence ranging from 20% to 71% among patients with TBI (20). A relatively high prevalence (57.9%) was observed in this sample, which again may reflect the characteristics of participants drawn from an outpatient psychiatric clinic. It should also be noted that 42% of patients presented with both depression and apathy, and 23.9% and 15.9% had only depression or only apathy, respectively. This might indicate that apathy and depression frequently co-occur among patients with TBI, and pure apathy, or depression without apathy, are less frequent.
By using hierarchical clustering, the component of apathy was successfully extracted from the item-to-item analyses of the BDI-II. This extracted “apathy cluster” of the BDI-II included six symptoms. Because some of the items, such as “loss of energy,” were common or related to those of the AS (34, 41), it is not surprising that the apathy subscore showed a robust correlation with the total AS score.
Although provisionally referred to here as an apathy cluster, the following conceptual issues should also be noted. First, among the six items included in this cluster, four are considered to be motivational symptoms, but the other two (“concentration difficulty” and “indecisiveness”) are more properly regarded as symptoms of subjective assessment of one’s own cognition. Second, among the four motivational symptoms, “loss of pleasure” and “loss of interest” are more appropriately considered anhedonia rather than apathy. Further conceptual and empirical fractionization within the apathy cluster is needed in future studies. Previous work has successfully distinguished apathy from depression among patients with depressive disorder or dementia (13, 16–18), but not among patients with TBI (26, 27). As noted earlier, the prevalence of apathy with depression was high among participants in this study, which may have facilitated successful separation of apathy from depression in this relatively small sample.
Moreover, the loss of self-worth subscore also correlated with the total AS score. Of the broader symptoms of depression, this cluster might be relatively similar to apathy. A somatic cluster was identified in this study, similar to a previous study that used a factor analysis of the BDI scores among patients with TBI (26). Indeed, changes in sleep, appetite, or libido may occur among patients with TBI, independent of depression (19). Thus, it is important to pay clinical attention to the symptoms in this cluster because they should be treated separately from psychiatric symptoms, especially among patients with TBI.
One of the novel findings of our study is that the apathy symptoms subscore was correlated with amount of daily activity, whereas the other subscores had no relationship with it. Depression includes not only symptoms that decrease activity but also symptoms that increase activity, such as agitation or irritability. In this study, the apathy component of the various depressive symptoms reported was associated with a decreased amount of activity. In addition, in a supplementary analysis of the effects of medication, no difference was found in the amount of activity between patients who were and were not taking medication. Therefore, decreased daily activity is not solely attributable to oversedation.
Among patients, apathy is often accompanied by a lack of insight (42). Thus, it is widely believed among clinicians that the self-assessment of apathy is unreliable, and objective assessments by clinicians or relatives are thus indispensable. However, the correlation with real-life activity supports the validity of subjective ratings in the assessment of apathy. Although self-reports may not be reliable for severe apathy, they may be able to make a fair estimation of real-life apathy symptoms among those with mildly to moderately severe apathy.
Our study has several limitations that should be considered. First, self-reported questionnaires to measure psychiatric symptoms and daily activity were used. Although the findings support the validity of using a subjective rating scale, it might not be suitable for those with more severe apathy, because the lack of insight might hinder reliable self-assessment. Moreover, in addition to lack of insight, the present analyses did not include neuropsychological impairments as covariates. Cognitive functions such as memory may affect the assessment of psychiatric symptoms. Future studies should seek to replicate or modify the present findings using other assessments of apathy. Second, because of the clinical settings of this study, most patients were in the chronic phase of TBI and likely to have psychiatric symptoms. Thus, the present findings may not generalize to the population with TBI as whole, particularly to those who are not receiving care in a psychiatric clinic. Hence, this study should be regarded as preliminary and should be reexamined among a larger sample that also includes patients with mild psychiatric symptoms. Third, patients with various types of injury were included in the present study, including persons with focal lesions or diffuse axonal injury; moreover, lesion locations among patients with focal injury were diverse. Therefore, the relationship between lesions and symptoms could not be studied in this relatively small sample of participants. Future studies are needed to address whether the clusters isolated from depressive symptoms have different structural or functional neuroanatomies.
Overall, the present findings support the thesis that apathy is clinically separable from broader depressive symptoms. On the basis of the present findings, decomposing heterogeneous depressive symptoms would be useful for selecting appropriate treatments for individual patients. This is important because the pharmacological options for treating apathy are different from those used to treat depression (11, 36, 43). Nonpharmacological interventions for apathy and depression are also fundamentally different. For example, as a general rule, patients with depression should not be overencouraged to do things: for patients with a substantially negative mood, dysphoria in particular, it is advisable to motivate them gently, delicately, and gradually in daily activities after maximal amelioration of the symptoms with medication. However, it is reasonable to encourage patients with apathy to participate in daily activities or rehabilitation in a more straightforward manner. Considering that neuropsychiatry specialists are not always available in many clinical settings, the use of self-rating scales might help to guide the choice of clinical intervention.
1 : Apathy in Parkinson’s disease. Int Rev Neurobiol 2017; 133:657–678Crossref, Medline, Google Scholar
2 : Apathy following traumatic brain injury. Psychiatr Clin North Am 2014; 37:103–112Crossref, Medline, Google Scholar
3 : Apathy and depression in mild Alzheimer’s disease: a cross-sectional study using diagnostic criteria. J Alzheimers Dis 2012; 31:325–334Crossref, Medline, Google Scholar
4 Quantifying motivational deficits and apathy: a review of the literature. Eur Neuropsychopharmacol. 2015;25(8):1060–1081Crossref, Medline, Google Scholar
5 : Management of depression in Parkinson’s disease: a systematic review. Mov Disord Clin Pract (Hoboken) 2017; 4:470–477Crossref, Medline, Google Scholar
6 : Depression and schizophrenia: cause, consequence, or trans-diagnostic issue? Schizophr Bull 2017; 43:240–244Medline, Google Scholar
7 : Pharmacological and non-pharmacological interventions of depression after traumatic brain injury: a systematic review. Eur J Pharmacol 2019; 865:172775Crossref, Medline, Google Scholar
8 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013Google Scholar
9 : Differential diagnosis and classification of apathy. Am J Psychiatry 1990; 147:22–30Crossref, Medline, Google Scholar
10 : Apathy is not depression. J Neuropsychiatry Clin Neurosci 1998; 10:314–319Link, Google Scholar
11 : Apathy in Alzheimer’s disease. J Am Geriatr Soc 2001; 49:1700–1707Crossref, Medline, Google Scholar
12 : Nonpharmacological and pharmacological interventions for symptoms in Alzheimer’s disease. Expert Rev Neurother 2004; 4:809–821Crossref, Medline, Google Scholar
13 : Principal components of the Beck Depression Inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol Psychiatry 2002; 51:387–399Crossref, Medline, Google Scholar
14 : Components of major depression examined via the Beck Depression Inventory. J Affect Disord 1992; 26:251–259Crossref, Medline, Google Scholar
15 : Apathy and depression: separate factors in Parkinson’s disease. J Int Neuropsychol Soc 2011; 17:1058–1066Crossref, Medline, Google Scholar
16 : Factor structure of the Geriatric Depression Scale and relationships with cognition and function in Alzheimer’s disease. Dement Geriatr Cogn Disord 2012; 34:360–372Crossref, Medline, Google Scholar
17 : Factor structure of the Brief Psychiatric Rating Scale in unipolar depression. J Affect Disord 2010; 124:329–334Crossref, Medline, Google Scholar
18 : A three-factor analytic model of the MADRS in geriatric depression. Int J Geriatr Psychiatry 2003; 18:73–77Crossref, Medline, Google Scholar
19 : Mood disorders following traumatic brain injury. NeuroRehabilitation 2002; 17:311–324Crossref, Medline, Google Scholar
20 Apathy following traumatic brain injury: a review. Neuropsychologia 2018;118(Pt B):40–47Crossref, Medline, Google Scholar
21 : Neuropsychiatric disorders in persons with severe traumatic brain injury: prevalence, phenomenology, and relationship with demographic, clinical, and functional features. J Head Trauma Rehabil 2011; 26:116–126Crossref, Medline, Google Scholar
22 : Psychiatric disturbances after traumatic brain injury: neurobehavioral and personality changes. Curr Psychiatry Rep 2006; 8:73–80Crossref, Medline, Google Scholar
23 : A multidimensional approach to apathy after traumatic brain injury. Neuropsychol Rev 2013; 23:210–233Crossref, Medline, Google Scholar
24 : Depression following traumatic brain injury: epidemiology, risk factors and management. CNS Drugs 2012; 26:111–121Crossref, Medline, Google Scholar
25 : Depression, apathy and impaired self-awareness following severe traumatic brain injury: a preliminary investigation. Brain Inj 2019; 33:1245–1256Crossref, Medline, Google Scholar
26 : The clinical utility of the Beck Depression Inventory after traumatic brain injury. Brain Inj 2001; 15:1021–1028Crossref, Medline, Google Scholar
27 : Use of the Beck Depression Inventory-II (BDI-II) with persons with traumatic brain injury: analysis of factorial structure. Brain Inj 2005; 19:77–83Crossref, Medline, Google Scholar
28 : Assessment of coma and impaired consciousness: a practical scale. Lancet 1974; 2:81–84Crossref, Medline, Google Scholar
29 : Complications of Mild Traumatic Brain Injury in Veterans and Military Personnel: A Systematic Review. Washington, DC, U.S. Department of Veterans Affairs, 2013Google Scholar
30 : Nizofenone administration in the acute stage following subarachnoid hemorrhage: results of a multi-center controlled double-blind clinical study. J Neurosurg 1986; 64:420–426Crossref, Medline, Google Scholar
31 : Difficulty and inaccuracy of assessment of the consciousness level by the Glasgow Coma Scale: comparison with the Japan Coma Scale. J Jpn Soc Emerg Med. 2007; 10:20–25Google Scholar
32 : Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 2006; 16:916–928Crossref, Medline, Google Scholar
33 : Cross-cultural validation of the Beck Depression Inventory-II in Japan. Psychiatry Res 2002; 110:291–299Crossref, Medline, Google Scholar
34 : Apathy following cerebrovascular lesions. Stroke 1993; 24:1625–1630Crossref, Medline, Google Scholar
35 : Assessment of motivational loss in poststroke patients using the Japanese version of Starkstein’s Apathy Scale. Nosotchu. 1998; 20:318–323Crossref, Google Scholar
36 : Prevalence of apathy following head injury. Brain Inj 1998; 12:87–92Crossref, Medline, Google Scholar
37 : Sex-specific regional grey matter volume correlates of daily activities. Sci Rep 2018; 8:9935Crossref, Medline, Google Scholar
38 : Manual for the Beck Depression Inventory-2. San Antonio, Tex., Psychological Corporation, 1996Google Scholar
39 : Association of depressive symptoms with functional outcome after traumatic brain injury. J Head Trauma Rehabil 2012; 27:87–98Crossref, Medline, Google Scholar
40 : The prevalence and symptom rates of depression after traumatic brain injury: a comprehensive examination. Brain Inj 2001; 15:563–576Crossref, Medline, Google Scholar
41 : Poststroke apathy and regional cerebral blood flow. Stroke 1997; 28:2437–2441Crossref, Medline, Google Scholar
42 : Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991; 38:143–162Crossref, Medline, Google Scholar
43 : Pharmacological management of the psychiatric aspects of traumatic brain injury. Int Rev Psychiatry 2003; 15:359–370Crossref, Medline, Google Scholar



