Embedded Psychiatric Services in a Multidisciplinary Amyotrophic Lateral Sclerosis Clinic: An Assessment of Patient Needs and Perceptions
Abstract
Objective:
Amyotrophic lateral sclerosis (ALS) is often associated with a range of difficult neuropsychiatric symptoms and conditions, including depression, apathy, pseudobulbar affect, and frontotemporal dementia (FTD). Despite the potential role for psychiatrists in the treatment of ALS, they are not typically involved in the ALS clinical team. The investigators describe a quality improvement intervention providing embedded psychiatric services within a multidisciplinary clinic (MDC).
Methods:
A psychiatrist working within an ALS MDC evaluated patients (N=116) over a 1-year period. The clinic assessed the prevalence of neuropsychiatric symptoms and conditions in patients with ALS (depression, anxiety, pseudobulbar affect, and cognitive impairment, including FTD) using standardized screening methods. Fifty-five patients and 47 family members completed surveys about perceptions of their need for psychiatric care, their experience of meeting with a psychiatrist, and their desire for future access to psychiatric care.
Results:
Prevalence rates for neuropsychiatric symptoms were 14.9% for depression, 11.3% for anxiety, 19% for cognitive impairment (including FTD, 8.6%), and 36.2% for pseudobulbar affect; 62.0% of patients were being prescribed at least one psychotropic medication. Both patients and family members reported that meeting with a psychiatrist was helpful, that the treatment provided was helpful, and that they would prefer continued availability of psychiatric services in the future. The presence of cognitive impairment and use of antidepressants increased the likelihood of patients reporting a benefit from psychiatric care.
Conclusions:
Patients with ALS report a benefit from increased access to psychiatric services. The inclusion of a psychiatrist within the ALS MDC model should be considered to improve quality of care for this patient population.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that predominantly affects upper and lower motor neurons, resulting in progressive muscle weakness, severe disability, and eventual ventilatory failure. ALS is incurable and almost always fatal, with a median survival of 2–3 years from symptom onset (1, 2). Neuropsychiatric symptoms and conditions, such as depression, anxiety, apathy, pseudobulbar affect, and frontotemporal dementia (FTD), are common in ALS (3). Psychiatric symptoms influence quality of life in multiple categories, including general health, vitality, social functioning, and emotional well-being (4–6). Higher burden of psychiatric symptoms may even be predictive of increased mortality (7).
The association of depression with ALS is well studied. The poor prognosis and significant disability from ALS likely contribute substantially to the development of depression; however, there seems to also exist an endogenous neurologic contribution from the disease process itself (3, 8). The prevalence of depression in ALS varies widely across different studies, due in part to the heterogeneity of study methods and measurement scales (3, 9, 10). The prevalence of depression has been estimated to be as high as 44%, although more modest estimates place the prevalence at 9%−11% (3, 10, 11). Research has demonstrated substantially higher antidepressant medication use in patients with ALS compared with the general population, as well as increasing antidepressant prescriptions in the months preceding and following ALS diagnosis (12). Although it is unclear how many of these prescriptions are for other indications besides depression (e.g., pain, sialorrhea, and sleep), the correlation between increased rates of depression and increased use of antidepressants in patients with ALS cannot be ignored (12, 13).
In addition to depression, pseudobulbar affect (PBA), characterized by episodes of uncontrollable crying, laughing, or other emotional outbursts, is common in patients with ALS, with an estimated prevalence of 20%–60%. The crying spells in PBA frequently overlap with symptoms of depression and may be difficult to differentiate (14, 15). Additionally, at least 10%–15% of patients with ALS develop FTD, resulting in significant cognitive impairment and behavioral disturbances (3, 16, 17). Cognitive-behavioral symptoms of FTD may include executive dysfunction, apathy, diminished social-emotional reciprocity, impulsivity, obsessions, and compulsions (3, 17, 18).
Symptom management is the primary form of intervention in ALS (1, 13). Increasingly, this is provided in a multidisciplinary clinic (MDC), which incorporates various specialties (e.g., neurology, physical and occupational therapy, speech therapy, respiratory therapy, nutrition, and neuropsychology) in a co-located, coordinated model of care (2, 19).The MDC model is associated with increased cost savings, survival, and quality of life and has become the gold standard for ALS treatment in the United States and other countries (19–22).
Psychologists, social workers, or other nonmedically trained mental health providers are frequently included among MDC providers to address the psychological and psychiatric needs of patients with ALS (19, 22, 23). Psychiatrists are typically not included in ALS MDC treatment teams, despite their potential contribution to the care of these patients. In addition to providing pharmacologic management for symptoms of depression and anxiety, psychiatrists can evaluate and treat more complex, overlapping neuropsychiatric conditions, such as apathy, PBA, and behavioral manifestations of FTD. Psychiatrists, particularly those with palliative care training or experience, may also provide expertise and support in assessing patient decisional capacity and navigating end-of-life discussions with patients and their caregivers.
Here, we describe a quality improvement intervention to increase access to psychiatric care for patients with ALS by providing embedded psychiatric services within a large ALS MDC. We assessed the prevalence of psychiatric needs among patients in the clinic and evaluated patient and family member perceptions of their psychiatric needs and the provision of psychiatric care. We hypothesized that patients with a higher burden of psychiatric symptoms would report increased satisfaction from having access to psychiatric services within the clinic.
METHODS
Setting and Participants
The South Texas ALS Clinic is an MDC operated by the Department of Neurology, University of Texas Health Science Center, San Antonio. It is one of 72 ALS Certified Treatment Centers of Excellence accredited by the ALS Association. Patients with ALS from across the South Texas region receive consultation and care from a neuromuscular neurologist, speech therapist, physical therapist, occupational therapist, respiratory therapist, dietician, and neuropsychologist in a co-located, coordinated model of care. During clinic appointments, patients rotate through each of the specialists by moving from room to room. At the initial intake, patients typically see every specialist; on follow-up visits, patients’ needs determine which specialists they see.
Beginning in August 2019, the South Texas ALS clinic added psychiatric services to the multidisciplinary team. Third-year psychiatric resident physicians (M.H. and C.C.) working directly with the clinic neurologist (C.J.) and with indirect supervisory support from an offsite attending psychiatrist conducted brief psychiatric evaluations for each patient. If the evaluation uncovered psychiatric needs, the psychiatrist saw patients again at subsequent clinic visits. The psychiatrist answered questions from patients and their families about neuropsychiatric symptoms and treatment options. In close collaboration with the clinic neurologist, he or she prescribed various psychotropic medications. If patients needed more extensive psychiatric care beyond what the clinic could offer, the psychiatrist helped facilitate a referral for ongoing psychiatric care or psychotherapy in the community.
Assessment
As part of the standard previsit assessment, the clinic implemented a standardized screening for depression and anxiety using the Hospital Anxiety and Depression Scale (HADS) (24). HADS is a well-validated instrument for screening for anxious and depressive symptoms in medically ill patients and is often used in ALS studies due to its reduced reliance on somatic and neurovegetative symptoms that may overlap with ALS symptomatology (9, 24, 25). Additionally, the clinic screened for symptoms of PBA using the Center for Neurologic Study–Lability Scale (CNS-LS) (26). Results of the screening assessments helped guide the psychiatric evaluation.
To assess patient and family perceptions of and satisfaction with the psychiatric services, we invited all patients and their family members to complete a voluntary survey at the conclusion of the clinic encounter. These surveys asked about the impact of depression, anxiety, or cognitive dysfunction (changes in memory, thinking, or concentration) on the patients’ quality of life. The surveys also asked whether patients and their families felt that meeting with a psychiatrist was beneficial and whether they would like mental health services to continue to be offered in the clinic in the future. Patients responded to questions about how much they agreed with each survey statement according to a 5-point Likert scale (strongly disagree, disagree, neutral, agree, and strongly agree). For ease of analysis, the Likert results corresponded to negative and positive numerical values, with neutral at zero.
We collected additional demographic and clinical data, including age, sex, time since symptom onset (in months), and whether the ALS was bulbar onset. We assessed the level of ALS-related disability at the time of evaluation using the ALS Functional Rating Scale–Revised (ALSFRS-R) (27). We used chart review to assess the presence of cognitive impairment (including FTD), antidepressant prescriptions, and dextromethorphan-quinidine prescriptions. FTD and other forms of cognitive impairment were based on assessments from clinic neuropsychologist using the Edinburgh and Behavioral ALS Screen (ECAS) and other neuropsychologic instruments (28). The institutional review board at the University of Texas Health Science Center, San Antonio, reviewed this study and determined it to be exempt. Thus, no written informed consent was required.
Protocol Change
In March 2020, the COVID-19 pandemic necessitated a temporary closure of the clinic. The clinic returned to full multidisciplinary services using telehealth in May 2020. Psychiatric evaluations occurred via an institution-approved video telehealth platform compliant with the Health Insurance Portability and Accountability Act (HIPAA), and data collection resumed at that point. Thereafter, the clinic gradually transitioned to a mix of in-person and telehealth patient encounters. For telehealth encounters, patients were administered a secure HIPAA-compliant web-based version of the symptom scales (HADS and CNS-LS) and patient-caregiver surveys in lieu of the paper versions used in clinic.
Statistical Analysis
We obtained summary statistics for baseline demographic data. We calculated arithmetic means and standard deviations for the Likert results from the patient and family member experience surveys. Paired t tests were used to compare results between patients and family members. Ordered logistic regressions were used to evaluate the associations between depression, anxiety, or cognitive dysfunction and the degree to which patients felt that meeting with a psychiatrist was helpful or whether they would like access to psychiatric services in the future. Ordered logistic regression was the best statistical model because of the ordinal dependent variable (Likert scale). Regressions were adjusted for age, sex, time since symptom onset, and use of antidepressant medications.
RESULTS
Data collection occurred between December 2019 and November 2020. Over the course of 12 months, 116 patients were evaluated. Of these, 55 (47.4%) completed the postvisit experience surveys. Patients’ baseline demographic characteristics are presented in Table 1. The average age was 64.1 years (SD=9.3), and 48.3% were female. The median time since symptom onset (at the time of evaluation) was 31.4 months. The mean ALSFRS-R score was 26.4 (SD=9.4), representing a moderate level of physical disability. Thirty-four percent of patients had bulbar-onset ALS, and 19% had evidence of cognitive impairment at the time of evaluation. Of those with cognitive impairment, nearly half (8.6% of the total sample) had a score on the ECAS consistent with a diagnosis of FTD. The prevalence of PBA, as measured by using the CNS-LS, was 36.2%. The prevalence of depression, based on the HADS depression subscale, was 14.9%; an additional 17.5% of patients had borderline or partial depressive symptoms. The prevalence of anxiety, based on the HADS anxiety subscale, was 11.3%; an additional 13.9% of patients had borderline anxious symptoms. At the time of evaluation, 48.3% were being prescribed an antidepressant, 25.9% were being prescribed dextromethorphan-quinidine, and 12.1% were being prescribed both an antidepressant and dextromethorphan-quinidine. The subset of patients who completed the postvisit experience survey did not differ significantly from the total patient sample, with the exception of a higher prevalence of antidepressant use (62%).
| Total sample (N=116) | Completed postvisit survey (N=55) | |||
|---|---|---|---|---|
| Characteristic | N | % | N | % |
| Age (years) (mean±SD) | 64.1 | 9.3 | 65.2 | 10.0 |
| Sex | ||||
| Female | 56 | 48.3 | 24 | 43.6 |
| Male | 60 | 51.7 | 31 | 56.4 |
| Time since symptom onset (months) (median, interquartile range) | 31.4 | 17.6–62.1 | 27.8 | 15.9–49.5 |
| ALSFRS-R score (mean±SD)a | 26.4 | 9.4 | 27.8 | 9.2 |
| Bulbar onset | 39 | 33.6 | 22 | 40.0 |
| Cognitive impairmentb | 22 | 19 | 10 | 18.2 |
| Frontotemporal dementia | 10 | 8.6 | 4 | 7.3 |
| Cognitive impairment without frontotemporal dementia | 12 | 10.3 | 6 | 10.9 |
| Current dextromethorphan-quinidine use | 30 | 25.9 | 15 | 27.3 |
| Current antidepressant use | 56 | 48.3 | 34 | 61.8 |
| Pseudobulbar affectc | 42 | 36.2 | 21 | 38.2 |
| Depressiond | ||||
| None | 77 | 67.5 | 37 | 67.3 |
| Borderline | 20 | 17.5 | 7 | 12.7 |
| Present | 17 | 14.9 | 9 | 16.4 |
| Anxietye | ||||
| None | 86 | 74.8 | 38 | 69.1 |
| Borderline | 16 | 13.9 | 9 | 16.4 |
| Present | 13 | 11.3 | 7 | 12.7 |
TABLE 1. Demographic and clinical characteristics of patients with amyotrophic lateral sclerosis (ALS) who received psychiatric services within a multidisciplinary clinic
The results of the patient and family member postvisit experience surveys are shown in Figures 1 and 2. Results for patients and their family members essentially mirrored each another. Both patients (mean=0.84 [SD=1.04]) and their family members (mean=0.72 [SD=1.07]) agreed that ALS was emotionally challenging, with no statistical difference between their responses (paired t test=0.78, df=44, p=0.44). Family members (mean=1.43 [SD=0.77]) were more likely than patients (mean=4.0 [SD=0.97]) to agree that treating the psychological aspects of ALS was as important as the physical symptoms (t=2.87, df=44, p<0.01). On average, patients disagreed or were neutral that depression (mean=−0.76 [SD=1.19]), anxiety (mean=−0.79 [SD=1.17]), and cognitive impairment (mean=−0.74 [SD=1.33]) had a negative impact on their quality of life. The subset of patients who screened positive for depression did report a negative impact of depression on their quality of life (mean=0.78 [SD=0.44]). The same was true for those who screened positive for anxiety (mean=0.86 [SD=0.38]) and cognitive impairment (mean=0.44 [SD=0.53]).
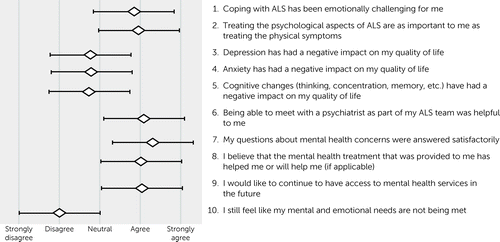
FIGURE 1. Results from a postvisit patient experience survey among patients with amyotrophic lateral sclerosis (ALS) who received psychiatric services within a multidisciplinary clinic (N=55)
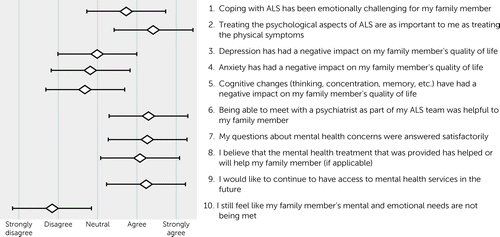
FIGURE 2. Results from a postvisit family member experience survey of psychiatric services within a multidisciplinary clinic (N=47)
On average, patients agreed that meeting with a psychiatrist was helpful to them (mean=1.1 [SD=0.92]), that their questions were answered satisfactorily (mean=1.23 [SD=0.75]), and that the treatment provided, if applicable, was helpful or would be helpful (mean=0.95 [SD=0.80]). They endorsed a desire for mental health services to be available to them in the future (mean=1.05 [SD=0.86]). Family members reported similar results, with no statistical difference between patients and family members (p>0.05 for all comparisons). Both patients and family members felt that the patient’s mental health needs were being met.
Table 2 displays the results of logistic regressions for the association between depression, anxiety, and cognitive impairment and perceived benefit of meeting with a psychiatrist, perceived benefit of treatment, and the desire for continued access to mental health services in the future. Neither the presence of depression nor anxiety had a statistically significant effect on the perceived benefit from meeting with a psychiatrist, the benefit of provided psychiatric treatment, or the desire for future mental health services. A diagnosis of cognitive impairment, on the other hand, did increase the likelihood of patients reporting a benefit from meeting with the psychiatrist (odds ratio=4.79, 95% CI=1.02–22.49, p=0.05). Antidepressant use was associated with an increased likelihood of reporting a benefit from the provided mental health treatment (odds ratio=4.04, 95% CI=1.04–15.70, p=0.04). Interestingly, the other factors of age, gender, and time since ALS diagnosis appeared to have minimal to no effect on patient perceptions of psychiatric services.
| Variable | Odds ratioa | 95% CI | pb |
|---|---|---|---|
| Psychiatrist as part of ALS team considered helpful (N=52) | |||
| Positive depression screen | 0.69 | 0.30, 1.59 | 0.39 |
| Positive anxiety screen | 1.63 | 0.62, 4.29 | 0.32 |
| Diagnosis of cognitive impairment | 4.79 | 1.02, 22.49 | 0.05 |
| Antidepressant use | 1.41 | 0.44, 4.59 | 0.56 |
| Time since symptom onset (months) | 1 | 0.99, 1.01 | 0.65 |
| Age (years) | 0.98 | 0.93, 1.03 | 0.45 |
| Male | 1.78 | 0.54, 5.89 | 0.34 |
| Psychiatric treatment provided considered helpful (N=50) | |||
| Positive depression screen | 0.58 | 0.24, 1.43 | 0.24 |
| Positive anxiety screen | 1.36 | 0.51, 3.60 | 0.54 |
| Diagnosis of cognitive impairment | 1.66 | 0.33, 8.24 | 0.54 |
| Antidepressant use | 4.04 | 1.04, 15.70 | 0.04 |
| Time since symptom onset (months) | 1 | 0.99, 1.01 | 0.97 |
| Age (years) | 0.96 | 0.91, 1.02 | 0.16 |
| Male | 2.79 | 0.75, 10.45 | 0.13 |
| Desire for continued access to psychiatric services in future (N=52) | |||
| Positive depression screen | 0.48 | 0.21, 1.11 | 0.09 |
| Positive anxiety screen | 1.84 | 0.69, 4.93 | 0.22 |
| Diagnosis of cognitive impairment | 3.19 | 0.63, 16.14 | 0.16 |
| Antidepressant use | 2.29 | 0.68, 7.72 | 0.18 |
| Time since symptom onset (months) | 0.99 | 0.99, 1.00 | 0.18 |
| Age (years) | 0.94 | 0.89, 0.99 | 0.03 |
| Male | 3.37 | 0.94, 12.12 | 0.06 |
TABLE 2. Amyotrophic lateral sclerosis (ALS) patient perceptions of psychiatric services within a multidisciplinary clinic
DISCUSSION
To our knowledge, this is the first study to explore the potential benefit of including a psychiatrist on the clinical team of an ALS MDC. Other studies have evaluated the benefit providing various psychological interventions to patients with ALS, usually in the form of brief cognitive-behavioral or mindfulness-focused therapies (23, 29). The provision of some form of psychological support to patients with ALS is generally accepted as an important component of the MDC model (18, 21). However, psychiatrists and other medically trained psychiatric providers may provide an important contribution above and beyond the psychological support provided by a psychologist, social worker, or other mental health counselor. Their medical training allows them to prescribe and manage psychotropic medications, understand and address the nuanced behavioral manifestations of FTD, and more effectively liaise with the neurologist to guide care. Working in tandem with psychologists, psychiatrists may provide added expertise to the diagnosis and management of the myriad neuropsychiatric symptoms in patients with ALS.
This study suggests that patients value the availability of psychiatric services, even when they are not currently experiencing any psychiatric symptoms. A large majority of patients felt that addressing the psychological aspects of ALS was as important as treating the physical symptoms of the disease. Furthermore, a large majority of the patients reported that being able to meet with a psychiatrist was helpful, that the treatment provided was helpful, and that they would like psychiatric services to be available to them in the future. Interestingly, the presence of depression and anxiety did not increase or decrease the likelihood of reporting a benefit from psychiatric services. The presence of cognitive impairment and FTD did increase the likelihood of reporting a benefit from meeting with the psychiatrist. The clinical significance of this finding is unclear but suggests that patients with cognitive impairment may especially benefit from psychiatric services.
Similar to patients, family members agreed that psychiatric services were helpful and wanted these services to be available in the future. Family members were included in this study in recognition of the important role they play in the care of patients with ALS. Family members are often acutely aware when their loved one is experiencing psychiatric symptoms related to ALS—sometimes more so than patients themselves—and are thus critical to the recognition of unmet needs. This is especially true when patients are experiencing anosognosia and are thus not fully aware of the severity of their neuropsychiatric symptoms, which is common in ALS (30).
This study has several limitations. First, data were collected from a single time point, rather than longitudinally. Thus, we are unable to comment on whether psychiatric services reduce depressive and anxiety symptoms in ALS patients over time. This initial study was intended to assess the need for, as well as patient-family member perceptions of, embedded psychiatric services in the ALS MDC. Future research should evaluate patients longitudinally to assess the effectiveness of embedded psychiatric services at reducing symptoms and improving quality of life. Additionally, this study did not include a control group, which would have provided further evidence of the effectiveness of providing embedded psychiatric services.
Another potential limitation is the generalizability of the model of care described in this study. The provision of psychiatric care within an ALS MDC may be limited by the availability of psychiatrists or lack of funding structures for providing psychiatric care. Admittedly, this model would work best in a large academic medical center where staff availability and the presence of grant funding may ameliorate these barriers. Having a psychiatrist physically present full-time in the ALS MDC may not be feasible; however, there are multiple ways in which psychiatrists could be involved in interdisciplinary collaboration with ALS treatment teams. For example, a psychiatrist could be available to provide virtual consults on particularly challenging cases.
In summary, this study suggests that expanding access to psychiatric care through the inclusion of a psychiatrist within an ALS MDC is both feasible and greatly appreciated by patients and their family members. The model of care described in this study can be adapted to other multidisciplinary clinical settings for ALS or other complex neurological conditions.
1 : Amyotrophic lateral sclerosis: an update for 2018. Mayo Clin Proc 2018; 93:1617–1628Crossref, Medline, Google Scholar
2 : Amyotrophic lateral sclerosis. Lancet 2011; 377:942–955Crossref, Medline, Google Scholar
3 : Psychiatric symptoms in amyotrophic lateral sclerosis: beyond a motor neuron disorder. Front Neurosci 2019; 13:175Crossref, Medline, Google Scholar
4 : Interaction of physical function, quality of life and depression in amyotrophic lateral sclerosis: characterization of a large patient cohort. BMC Neurol 2015; 15:84Crossref, Medline, Google Scholar
5 : The relationships between symptoms, disability, perceived health and quality of life in amyotrophic lateral sclerosis/motor neuron disease. Amyotroph Lateral Scler Frontotemporal Degener 2019; 20:317–327Crossref, Medline, Google Scholar
6 : Do pain, anxiety and depression influence quality of life for people with amyotrophic lateral sclerosis/motor neuron disease? A national study reconciling previous conflicting literature. J Neurol 2020; 267:607–615Crossref, Medline, Google Scholar
7 : Depression in ALS in a large self-reporting cohort. Neurology 2016; 87:1631–1632Crossref, Medline, Google Scholar
8 : Depression in amyotrophic lateral sclerosis. Neurology 2016; 86:2271–2277Crossref, Medline, Google Scholar
9 : Depression and anxiety in individuals with amyotrophic lateral sclerosis: a systematic review. Trends Psychiatry Psychother 2016; 38:1–5Crossref, Medline, Google Scholar
10 : Depression and anxiety in individuals with amyotrophic lateral sclerosis: epidemiology and management. CNS Drugs 2007; 21:279–291Crossref, Medline, Google Scholar
11 : Prevalence of fatigue and depression in ALS patients and change over time. J Neurol Neurosurg Psychiatry 2009; 80:1146–1149Crossref, Medline, Google Scholar
12 : The use of antidepressant medication before and after the diagnosis of amyotrophic lateral sclerosis: a population-based cohort study. Neuroepidemiology 2015; 44:91–98Crossref, Medline, Google Scholar
13 : A survey of clinicians’ practice in the symptomatic treatment of ALS. Amyotroph Lateral Scler Other Motor Neuron Disord 2003; 4:258–263Crossref, Medline, Google Scholar
14 : PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One 2013; 8:e72232Crossref, Medline, Google Scholar
15 : Laughter, crying and sadness in ALS. J Neurol Neurosurg Psychiatry 2017; 88:825–831Crossref, Medline, Google Scholar
16 : Cognitive, emotional and psychological manifestations in amyotrophic lateral sclerosis at baseline and overtime: a review. Front Neurosci 2019; 13:951Crossref, Medline, Google Scholar
17 : Dysexecutive functioning in ALS patients and its clinical implications. Amyotroph Lateral Scler Frontotemporal Degener 2015; 16:160–171Crossref, Medline, Google Scholar
18 : Apathy is associated with poor prognosis in amyotrophic lateral sclerosis. Eur J Neurol 2016; 23:891–897Crossref, Medline, Google Scholar
19 : Amyotrophic lateral sclerosis: improving care with a multidisciplinary approach. J Multidiscip Healthc 2017; 10:205–215Crossref, Medline, Google Scholar
20 : The benefit of evolving multidisciplinary care in ALS: a diagnostic cohort survival comparison. Amyotroph Lateral Scler Frontotemporal Degener 2017; 18:569–575Crossref, Medline, Google Scholar
21 : Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996-2000. J Neurol Neurosurg Psychiatry 2003; 74:1258–1261Crossref, Medline, Google Scholar
22 : Developing multidisciplinary clinics for neuromuscular care and research. Muscle Nerve 2017; 56:848–858Crossref, Medline, Google Scholar
23 : Effect of a short-term psychological intervention on the anxiety and depression of amyotrophic lateral sclerosis patients. J Health Psychol 2016; 21:1426–1435Crossref, Medline, Google Scholar
24 : The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res 2002; 52:69–77Crossref, Medline, Google Scholar
25 : Prevalence of major depression in ALS: comparison of a semi-structured interview and four self-report measures. Amyotroph Lateral Scler 2011; 12:297–302Crossref, Medline, Google Scholar
26 : A self report measure of affective lability. J Neurol Neurosurg Psychiatry 1997; 63:89–93Crossref, Medline, Google Scholar
27 : The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci 1999; 169:13–21Crossref, Medline, Google Scholar
28 : The Edinburgh Cognitive and Behavioral ALS Screen (ECAS) in frontotemporal dementia. Amyotroph Lateral Scler Frontotemporal Degener 2020; 21:606–613Crossref, Medline, Google Scholar
29 : Meditation training for people with amyotrophic lateral sclerosis: a randomized clinical trial. Eur J Neurol 2017; 24:578–586Crossref, Medline, Google Scholar
30 : Anosognosia in amyotrophic lateral sclerosis: a cross-sectional study of 85 individuals and their relatives. Ann Phys Rehabil Med (Online ahead of print, October 13, 2020) doi: 10.1016/j.rehab.2020.08.004Google Scholar



