Relationship of Cognitive Functioning, Adaptive Life Skills, and Negative Symptom Severity in Poor-Outcome Geriatric Schizophrenia Patients
Abstract
The authors assessed whether cognitive functioning and negative symptoms are related to functional outcome across severity of negative symptoms and examined relationships between symptom domains in patients with high versus low negative symptom severity. The interrelationships between cognitive functioning and functional skills in poor-outcome geriatric schizophrenic patients were compared between those who were in the first (n=81) and the fourth quartiles (n=127) of negative symptom severity based on the normative data in the Positive and Negative Syndrome Scale. It was found that negative symptoms and cognitive functioning were the strongest correlates of functional status in geriatric poor-outcome schizophrenic patients—regardless of negative symptom severity. Interestingly, the greater the severity of negative symptoms, the less strongly negative symptoms were related to functional outcome. The present findings demonstrate that the relationship of cognitive functioning to social and adaptive functioning remains significant despite differing levels of negative symptom severity.
Impairments in social and adaptive functioning are central features of schizophrenia. Cognitive dysfunction and negative symptoms, both of which are often enduring and resistant to conventional treatments, are present in most persons with schizophrenia. Past research has demonstrated that the degree of impairment in social and adaptive functioning is correlated with the severity of cognitive dysfunction and negative symptoms.
Prior cross-sectional studies of chronically hospitalized patients with poor outcome have demonstrated significant correlations between both negative and cognitive symptoms and impaired adaptive life skills,1–3 but these studies have the potential limitation of cohort effects. There are several ways to avoid these potential biases. Longitudinal evaluations of a single population help to control for potentially confounding factors and allow for the examination of the differential contributions of negative versus cognitive symptoms to adaptive functioning deficits. For example, moderate levels of cognitive and adaptive decline have been demonstrated in chronically institutionalized geriatric schizophrenic patients over a 2.5-year period culminating in referral to nursing home care.4 In these patients, cognitive and adaptive declines were intercorrelated and were not associated with the baseline severity of either positive or negative symptoms. Because positive and negative symptoms did not worsen over the follow-up period, there was no possibility of their predicting declines in adaptive functioning. Similarly, the use of subsamples of patients stratified on the basis of external criteria (such as normative standards for symptom severity) can also address these cohort effects.
Cognitive deficits and negative symptoms appear to be correlated in their severity. Yet it is possible that despite being correlated, cognitive dysfunction and negative symptoms represent the behavioral manifestations of different underlying physiological processes. In two recent longitudinal studies, Harvey et al.5 and McGurk et al.6 found substantial cross-sectional correlations between severity of negative symptoms and severity of cognitive symptoms at two successive assessments separated by one year, but no longitudinal relationship between these aspects of the illness. These data suggest the possibility of some alternative influence that determines the severity of both of these aspects of the illness.
An approach to better understanding the relationship between cognitive and negative symptoms and functional outcome would be to evaluate these relationships in patients within varying degrees of negative symptoms. It is not known if the relationships between negative symptoms, cognitive functioning, and adaptive life skills are dependent on the level of severity of negative symptoms. It is possible, for example, that patients with extreme scores on negative symptoms, aspects of cognitive performance, and adaptive functioning account for the correlation between these aspects of the illness in large samples of patients. A comparison of relationships between negative symptoms, cognitive performance, and adaptive life skills in individuals who have extreme versus minimal severity of one of these dimensions would address this question. If the correlates of functional status are the same in both groups, extreme scores in either direction cannot account for the overall pattern of relationships.
The intent of this study was to assess whether the relationships between cognitive functioning, clinical symptom severity, and functional outcome are similar in groups of patients that differ in negative symptom severity, and to examine these relationships within each subgroup. In this study, patients were separated into groups having high or low negative symptom severity based on normative criteria. These patient groups were then compared on the severity of other symptoms of the illness, including cognitive, positive, and functional aspects. Finally, the relationships of these different dimensions of illness were compared in the two negative symptom severity groups. If the relationships between negative symptoms, cognitive functioning, and adaptive life skills were found to be the same, then that would suggest that high versus low levels of negative symptoms define subgroups of patients that are essentially the same in terms of the parametric relationships between the different aspects of illness.
METHODS
Subjects
A total of 208 inpatients (94 males, 114 females) with a lifetime diagnosis of schizophrenia met inclusion criteria for this study. The mean age of the patients was 71.9 years (SD=11.4), the mean age at first hospitalization was 27.9 years (SD=12.3), and the mean number of years of education was 10.6 (SD=2.9). The low negative-symptom severity group had 55 female and 26 male subjects. The high negative-symptom severity group had 59 female and 68 male subjects. The vast majority of subjects were receiving typical antipsychotic medications.
All patients assented to participation, and the Internal Review Boards at Pilgrim Psychiatric Center and Mount Sinai School of Medicine approved a waiver of a signed informed consent. All patients included in this study were participants in a large-scale program of research on cognitive functioning and clinical symptoms in geriatric chronic psychiatric inpatients. In that study, the entire population of a state psychiatric center was rediagnosed and reevaluated with a comprehensive assessment of clinical, cognitive, and functional status. Research staff members performed diagnostic assessments, and a structured consensus procedure was employed to generate the DSM-III-R diagnosis of schizophrenia. The entire assessment procedure for that study has been published, and all subjects in the present study were diagnosed and assessed with that procedure.7,8
Potential participants were excluded from this study if they were in active treatment for a seizure disorder, if they had experienced a cerebral vascular accident; if they had a prior diagnosis of alcohol/drug dependence; if they had experienced a head trauma with loss of consciousness in the past; or if they had a concurrent pervasive developmental disorder, mental retardation, neurological diseases or damage, or other psychiatric diagnoses. All patients received an annual physical and neurological examination. Any patients who a neurologist determined, on the basis of a clinical evaluation, had experienced “rapid cognitive decline” in the past year were also excluded.
Assessments
Demographic data and information related to the functional abilities of each subject were obtained through a thorough review of each patient's medical chart and interview with staff members who are instrumental in the daily care of the patient. Severity of schizophrenic symptoms was assessed with the Positive and Negative Syndrome Scale (PANSS). Cognitive functioning was assessed with the Consortium for the Establishment of a Registry for Alzheimer's Disease (CERAD) battery,9 which included measures of word list learning and recall, praxic drawings, word naming, and category fluency. In previous analyses of this battery1,4,6 we have developed a composite measure of cognitive functioning, which was created by standardizing all of the scores within the sample of schizophrenic patients and averaging them together into a single score. Adaptive life skills were assessed with the Social Adaptive Functioning Evaluation (SAFE) scale.10 Description, administration procedures, and scoring of these assessments are described in detail in other publications from this group.7,8,11
Data analysis was conducted in two stages. First, the negative symptom severity groups were compared on demographic variables such as age at the assessment, age at first hospitalization, and number of years of education attained, as well as on tests of cognitive performance, functional outcomes, and symptom severity. Second, the relationships between these variables were evaluated both within and between high and low negative-symptom severity groups.
RESULTS
Demographic Characteristics
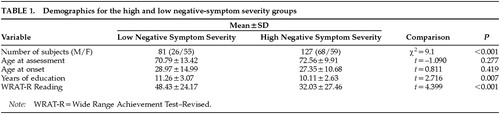
Subjects were grouped into high and low negative-symptom severity groups based on their PANSS Negative Symptom total scores. These groups were selected to include those patients who were in the first and fourth quartiles of negative symptom severity based on the normative data in the PANSS manual (25th percentile is a maximum total score of 18, and 75th is a minimum score of 26). Thus, this division is based on the PANSS norms and not the distribution of scores for this sample. The middle two quartiles were eliminated from this analysis to concentrate on the extreme groups in terms of the severity of negative symptoms. Age, age at onset, and years of education were compared across the two groups with a series of t-tests. (Table 1 displays means and standard deviations.) There were no group differences on age at time of the assessment (t=–1.090, P=0.277) or age at first psychiatric admission (t=0.811, P=0.419). The low negative-symptom severity group had significantly more education (t=2.716, P=0.007) and better Reading subtest scores on the Wide Range Achievement Test–Revised (t=4.399, P<0.001) than the high-symptom group.
A series of t-tests were performed to evaluate group differences on all of the cognitive and clinical measures. (Table 2 displays means and standard deviations.) There were group differences in positive symptom severity (t=–4.168, P<0.001), with the high negative-symptom severity group having significantly more positive symptoms. A multivariate analysis of covariance (MANCOVA), with education and gender as the covariates, demonstrated that the overall univariate effect remained significant for positive symptom severity (F=15.024, df=1,180, P<0.001).
There were also group differences on every cognitive measure assessed, with the high negative-symptom severity group performing more poorly than the low negative-symptom severity group. There were group differences on the MMSE (t=7.525, P<0.001), word list learning (t=6.570, P<0.001), delayed recall (t=4.244, P<0.001), praxic ability (t=5.247, P<0.001), confrontation naming (t=4.867, P<0.001), phonological fluency (t=6.649, P<0.001), and category fluency (t=5.931, P<0.001). When education and gender were controlled for by use of a MANCOVA, the overall multivariate effect was significant for cognitive performance when all cognitive variables were considered at once (Rao's R=7.014, df=7,174; Wilks' lambda=0.780; P<0.001). Results from the MANCOVA also demonstrated that the high negative-symptom severity group performed significantly more poorly on every cognitive test (see Table 2). There were group differences on the Mini-Mental State Examination (F=37.295, df=1,180, P<0.001), word list learning (F=25.177, df=1,180, P<0.001), delayed recall (F=8.622, df=1,180, P<0.01), praxic ability (F=24.097, df=1,180, P<0.001), confrontation naming (F=19.859, df=1,180, P<0.001), phonological fluency (F=26.985, df=1,180), and category fluency (F=25.657, df=1,180, P<0.001).
On the SAFE scale, the low negative-symptom severity group had significantly better scores for Self Care (t=–9.570, P<0.001), Social Functioning (t=19.262, P<0.001), Impulse Control (t=–5.323, P<0.001), and overall functioning as measured by the total SAFE scale score (t=–14.958, P<0.001). Again, when education and gender were controlled for with a MANCOVA, the overall multivariate was significant for all of the SAFE subscales considered at once (Rao's R=93.752, df=3,178; Wilks' lambda=0.388; P<0.001). After education and gender were controlled for, the low negative-symptom severity group still demonstrated significantly better scores for Self Care (F=76.667, df=1,180, P<0.001), Social Functioning (F=273.134, df=1,180, P<0.001), and Impulse Control (F=26.259, df=1,180, P<0.001), as well as overall functioning (F=176.43, df=1,180, P<0.001), even after education and gender were accounted for (see Table 2).
Correlations Between Adaptive Functioning, Cognitive Functioning, and Clinical Symptoms
Table 3 presents Pearson correlations between scores on the composite cognitive battery, total PANSS positive and negative scale scores, SAFE scale total scores, and SAFE scale component scores for each negative-symptom severity group. As can be seen in the table, the severity of positive symptoms was essentially uncorrelated with negative symptoms, cognitive functioning, and social and adaptive functioning in the high negative-symptom severity group. Positive symptom severity was correlated with impulse control in this group. In the low negative-symptom severity group, where negative symptoms were relatively absent (PANSS Negative Symptom Total<18), positive symptoms were significantly correlated with social functioning, impulse control, and negative symptom severity. In both negative-symptom groups, cognitive functioning and negative symptom severity were significantly correlated with social and adaptive functioning. Cognitive functioning was not related to impulse control in the low negative-symptom severity group but was significantly correlated with impulse control in the high negative-symptom severity group.
A structural equation analysis was performed to examine whether the overall patterns of correlation were similar for the two groups. This analysis tests the null hypothesis that the two matrices are sampled from the same population, with the test statistic being the chi-square test. A significant chi-square statistic indicates that two matrices are significantly different from each other; a nonsignificant result indicates that the null hypothesis of no difference cannot be rejected. This analysis was performed with the EQS version 2.0 program12 and produced a significant result (χ2=161.740, df=36, P<0.001). This result indicated that the overall correlation matrices are different for the two groups, suggesting differences in the overall structure of symptomatology for the two samples. Because the two correlation matrices were statistically significantly different, the magnitudes of the correlations were compared between the low and high negative-symptom severity groups in order to determine where the correlation matrices differed. There were no significant differences in the correlation magnitudes of any of the variables, however, when compared across negative-symptom severity groups.
Analysis of the Association of Cognitive Functioning and Clinical Symptom Status and Adaptive Functioning
Because the overall pattern of correlation differed across the groups, our interest became identifying the predictors of social and adaptive functioning within each group. Two regression analyses were run to identify the predictors of global social and adaptive functioning (SAFE Total Score), within each group. For each group, after the cognitive composites were derived, a simultaneous regression analysis was used to determine if there was a significant overall relationship between the cognitive and clinical variables and social and adaptive functioning. If there was a significant result, the variables were reexamined in a stepwise regression analysis. For the low negative-symptom severity group, the simultaneous regression analysis of the effects of positive symptom severity (PANSS Positive Symptom Total), negative symptom severity (PANSS Negative Symptom Total), and cognitive functioning (Cognitive Composite) on social and adaptive functioning (SAFE Total Score) was significant (F=10.08, df=3,77, P<0.001; R2=0.282). Because this result was significant, the stepwise regression analysis was performed to determine which of the predictor variables accounted for the variance in global social and adaptive functioning. The analysis was statistically significant at the first step, with negative symptom severity entering into the equation (F=15.81, df=1,79, P<0.001; R2=0.17). At step 2 of the analysis, cognitive functioning entered into the equation, and the overall analysis was statistically significant (F=13.20, df=2,78, P<0.001; incremental R2=0.08). Positive symptom severity did not account for a statistically significant additional portion of the variance in social and adaptive functioning for this group.
The same regression procedure was conducted for the high negative-symptom severity group (PANSS Negative Symptom Total>26). In this analysis, the simultaneous regression analysis of the effects of positive symptom severity, negative symptom severity, and cognitive functioning on social and adaptive functioning was significant (F=30.471, df=3,123, P<0.001; R2=0.43). In the stepwise regression analysis, the analysis was significant at the first step, with cognitive functioning entering into the equation (F=63.074, df=1,125, P<0.001; R2=0.34). At step 2 of the analysis, negative symptom severity entered into the equation, and the overall analysis was statistically significant (F=43.134, df=2,124, P<0.001; incremental R2=0.08). Positive symptom severity did not account for a statistically significant additional portion of the variance in social and adaptive functioning for this group.
Analysis of the Effects of Cognitive Functioning and Symptom Status on Individual Domains of Functional Status
To evaluate the effects of symptomatology and cognitive functioning on individual functional domains from the SAFE scale (self-care ability, social functioning, and impulse control), the same series of regression analyses were run to identify the best predictors for each domain.
Self-Care:
For the low negative-symptom severity group, the simultaneous regression analysis of the effects of positive symptom severity, negative symptom severity, and cognitive functioning on self-care ability (SAFE Self-Care Score) was significant (F=7.676, df=3,77, P<0.001; R2=0.230). In the stepwise regression analysis, the analysis was statistically significant at the first step, with cognitive performance entering into the equation (F=15.318, df=1,79, P<0.001; R2=0.16). At step 2 of the analysis, negative symptom severity entered into the equation, and the overall analysis was statistically significant (F=11.480, df=2,78, P<0.001; incremental R2=0.07). Positive symptom severity did not account for a statistically significant additional portion of the variance in self-care ability for this group.
In the analysis for the high negative-symptom severity group, the simultaneous regression analysis was significant (F=22.148, df=3,123, P<0.001; R2=0.35). In the stepwise regression analysis, the analysis was statistically significant at the first step, with cognitive functioning entering into the equation (F=56.898, df=1,125, P<0.001; R2=0.31). At step 2 of the analysis, negative symptom severity entered into the equation, and the overall analysis was statistically significant (F=33.367, df=2,124, P<0.001; incremental R2=0.04). Positive symptom severity did not account for a statistically significant additional portion of the variance in self-care ability for this group.
Social Functioning:
For the low negative-symptom severity group, the simultaneous regression analysis was significant (F=9.08, df=3,77, P<0.001; R2=0.26). In the stepwise regression analysis, the analysis was significant at the first step, with negative symptom severity entering into the equation (F=17.69, df=1,79, P<0.001; R2=0.18). At step 2 of the analysis, positive symptom severity entered into the equation, and the overall analysis was significant (F=11.778, df=2,78, P<0.001; incremental R2=0.04). Cognitive functioning did not account for a significant additional portion of the variance in social functioning for this group.
For the high negative-symptom severity group, the simultaneous regression analysis was significant (F=24.32, df=3,123, P<0.001; R2=0.37). In the stepwise regression analysis, the analysis was significant at the first step, with cognitive functioning entering into the equation (F=41.696, df=1,125, P<0.001; R2=0.25). At step 2 of the analysis, negative symptom severity entered into the equation, and the overall analysis was significant (F=34.15, df=2,124, P<0.001; incremental R2=0.11). Positive symptom severity did not account for a significant additional portion of the variance in social functioning for this group.
Impulse Control:
For the low negative-symptom severity group, the simultaneous regression analysis was significant (F=3.175, df=3,77, P<0.05; R2=0.110). In the stepwise regression analysis, the analysis was statistically significant at the first step, with positive symptom severity entering into the equation (F=7.40, df=1,79, P<0.01, R2=0.09). Neither cognitive functioning nor negative symptom severity accounted for a significant additional portion of the variance in impulse control for this group.
For the high negative-symptom severity group, the simultaneous regression analysis was significant (F=13.08, df=3,123, P<0.001; R2=0.24). In the stepwise regression analysis, the analysis was significant at the first step, with positive symptom severity entering into the equation (F=18.54, df=1,125, P<0.001; R2=0.13). At step 2 of the analysis, cognitive functioning entered into the equation (F=18.48, df=2,124, P<0.001; incremental R2=0.10). Negative symptom severity did not account for a significant additional portion of the variance in impulse control for this group.
DISCUSSION
This study demonstrated that cognitive functioning is the strongest correlate of functional status in geriatric poor-outcome schizophrenic patients, regardless of negative symptom severity. Furthermore, cognitive functioning was related to adaptive functioning across levels of severity of negative symptoms, although in patients with lower levels of negative symptoms the relationship was weaker.
Past research with this poor-outcome population demonstrated significant relationships between cognitive functioning and various indices of social and adaptive functioning, but there was also a significant relationship of these measures with negative symptom severity. Therefore, it was previously unclear whether negative symptoms mediated the relationship between cognitive functioning and functional skills, since patients who exhibit a relatively severe level of negative symptoms might perform poorly on tests of cognitive functioning not because they are cognitively impaired, but rather as a result of apathy and lack of involvement with the assessment procedure.
The relevance of the present findings is that they demonstrate that the relationship of cognitive functioning and social and adaptive functioning remains significant despite differing levels of negative symptom severity. Interestingly, the greater the levels of negative symptomatology, the less strongly negative symptoms were related to functional outcome. Therefore, the relationship between cognitive functioning and social and adaptive outcome is not mediated by an apathetic test-taking approach, but rather by the cognitive deficits themselves.
There are several limitations to this study that may influence the interpretation of the findings. First, these are very elderly poor-outcome patients whose characteristics may be different from those of patients who are younger and have a better lifetime functional outcome. Second, the low and high negative-symptom groups were selected because of extreme scores on the PANSS negative symptom subscale. As a result, the variance of these scores is intrinsically reduced, and correlations between negative symptom scores and other variables may be reduced as well. This confound is mitigated, however, by the similarity of the present results to those of past research with this population in which there was no range restriction on this variable.13
The finding that the relative presence or absence of negative symptoms does not eliminate the relationship between negative symptoms and adaptive life skills suggests, however, that negative symptom severity is associated with functional outcome in schizophrenia. Even in patients with the lower levels of negative symptom severity, negative symptoms predicted adaptive deficits. These findings are in contrast to those in previous studies where negative symptoms did not predict the severity of adaptive deficits when cognitive functions were considered.
One of the issues that must be considered in this study is that of the definition of negative symptoms. The PANSS considers symptoms such as “deficits in abstract thinking” to be negative symptoms. Clinical ratings of such symptoms could not be considered to be independent of cognitive test performance. The field must decide how to measure cognitive and behavioral symptoms and draw some distinction between them. If we do not distinguish these symptoms, then “correlations” between symptom dimensions may lead to artificial searches for biological relationships between variables that are simply related on the basis of definition alone.
Another issue these data elucidate is the role of motivation in cognitive test performance. For example, patients with severe negative symptoms are often seen as being less motivated, perhaps explaining poorer cognitive performance. However, the present findings demonstrate that cognitive functioning accounted for greater variance in total functional outcome with greater levels of severity of negative symptoms. This underscores the fact that performance-based measures of cognition are not a product of poor motivation during cognitive evaluation.
Regardless of negative symptom severity, positive symptom severity was most strongly related to the severity of impulsive behavior. These findings replicate earlier studies that show that cognition and negative symptom severity are more closely related to failure to initiate purposeful behaviors, and positive symptom severity is more related to failure to control impulsive behavior. Interestingly, the relationship between clinical symptoms and instrumental life skills (self-care and social functioning) varied as a function of negative symptom severity. In patients with relatively less severe negative symptoms, both negative and positive symptoms were significantly associated with social functioning, whereas cognitive functioning was not. In contrast, cognitive functioning was most associated with impaired social functioning in patients with relatively high negative symptom severity.
Because these data suggest that cognitive functioning has significant relationships with independent living skills, regardless of the severity of negative symptoms, the most efficacious approach to improving outcome would be to target cognition dysfunction. Novel antipsychotic medications, such as clozapine, risperidone, olanzapine, and quetiapine, have shown promise in their ability to improve cognitive performance, particularly in those domains that have been shown to relate to functional ability. For example, clozapine, risperidone, and olanzapine have all been shown to improve verbal learning and memory, problem-solving skills, and attention (see review14). Because these cognitive domains have been shown to be important in successful community living,15 treatments that include the use of these medications may improve the ability to function in a community setting.
ACKNOWLEDGMENTS
These findings were presented at the International Congress for Schizophrenia Research, Santa Fe, NM, April 18–22, 1999, and the American Psychiatric Association annual meeting, Washington, DC, May 16–20, 1999.
 |
 |
 |
1 Harvey PD, Powchik P, Parrella M, et al: Symptom severity and cognitive impairment in chronically hospitalized geriatric patients with mood disorders. Br J Psychiatry 1997; 170:369–374Crossref, Medline, Google Scholar
2 Harvey PD, Sukhodolsky D, Parrella M, et al: The association between adaptive and cognitive deficits in geriatric chronic schizophrenic patients. Schizophr Res 1997; 27:211–218Crossref, Medline, Google Scholar
3 Harvey PD, Howanitz E, Parrella M, et al: Cognitive, adaptive, and clinical symptoms in geriatric patients with lifelong schizophrenia: a comparative study across treatment sites. Am J Psychiatry 1998; 155:1080–1086Google Scholar
4 Harvey PD, Parrella M, White L, et al: The convergence of cognitive and adaptive decline in late-life schizophrenia. Schizophr Res 1999; 35:77–84Crossref, Medline, Google Scholar
5 Harvey PD, Lombardi J, Leibman M, et al: Cognitive impairment and negative symptoms in schizophrenia: a prospective study of their relationship. Schizophr Res 1996; 22:223–231Crossref, Medline, Google Scholar
6 McGurk SR, Moriarty PJ, Harvey PD, et al: The longitudinal relationship of clinical symptoms, cognitive functioning, and adaptive life skills in geriatric schizophrenia. Schizophr Res 2000; 42:47–55Crossref, Medline, Google Scholar
7 Davidson M, Harvey PD, Powchik P, et al: Severity of symptoms in geriatric chronic schizophrenic patients. Am J Psychiatry 1995; 152:197–207Crossref, Medline, Google Scholar
8 Davidson M, Harvey PD, Welsh KA, et al: Characterization of the cognitive impairment of old-age schizophrenia: a comparison to patients with Alzheimer's disease. Am J Psychiatry 1996; 153:1274–1279Google Scholar
9 Morris JC, Heyman A, Mohs RC, et al: The Consortium to Establish a Registry for Alzheimer's Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989; 39:1159–1165Google Scholar
10 Harvey PD, Davidson M, Mueser KT, et al: The Social Adaptive Functioning Evaluation: an assessment measure for geriatric psychiatric patients. Schizophr Bull 1997; 23:131–146Crossref, Medline, Google Scholar
11 Harvey PD, Silverman J, Mohs RC, et al: Cognitive functioning in late-life schizophrenia: a longitudinal study of geriatric chronically hospitalized patients. Biol Psychiatry 1999; 45:32–40Crossref, Medline, Google Scholar
12 Bentler PM, Mooijaart A: Choice of structural model via parsimony: rationale based on precision. Psychol Bull 1989; 106:315–317Crossref, Medline, Google Scholar
13 Moriarty PJ, Lieber DG, Bennett A, et al: Gender differences in poor outcome patients with lifelong schizophrenia. Schizophr Bull (in press)Google Scholar
14 Meltzer HY, McGurk SR: The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 1999; 25:233–255Crossref, Medline, Google Scholar
15 Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321—330Google Scholar



