Acquired Obsessive-Compulsive Disorder Associated With Basal Ganglia Lesions
Abstract
The authors report 5 cases of acquired obsessive-compulsive disorder occurring later in life. Patients' presentations, which could be readily mistaken for a delusional disorder, were associated with depressive symptoms and basal ganglia lesions, implicating dysfunction of the cortical–basal ganglia–thalamic–cortical neuroanatomical circuit.
Obsessive and compulsive behaviors (OCBs), most often present in the context of idiopathic obsessive-compulsive disorder (OCD), are typically well developed by the second or third decade.1 However, there are numerous reports of acquired OCD or OCBs that have a precipitous onset, occurring secondary to brain lesions.2,3 Localization of brain pathology in these acquired cases spans the anatomical spectrum from diffuse involvement2 to concentration in focal areas such as basal ganglia.3
Neuroanatomical models for obsessive-compulsive symptoms have been proposed.4–6 Information gathered from neuroimaging studies suggests that a cortical–basal ganglia–thalamic–cortical (CBGTC) axis composes this circuit.7–11 Several studies have also attempted to identify neuropsychological correlates involving frontal and/or visuospatial impairments.12,13
In this article we describe 5 cases of late-onset acquired OCD, carefully examined from psychiatric, neuroimaging, and neuropsychological standpoints, and the important treatment implications of accurate diagnosis. In particular, the fixed nature of the acquired obsessions we observed can place patients such as these at risk for misdiagnosis of a psychotic delusional disorder and inappropriate treatment with potentially harmful neuroleptics.
PATIENTS
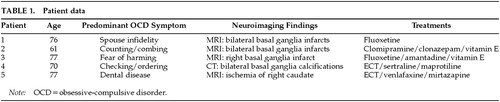
The patients are 5 women ranging in age from 61 to 77. Four are high school graduates and one has had 2 years of college. Four have histories of depression beginning at or after age 60, but none had histories of obsessive-compulsive disturbances. None were manifesting memory impairment or adaptive deficiencies indicative of a dementia. Each of our patients has basal ganglia lesions that were found after the development of their obsessions and compulsions. Table 1 provides data regarding patients' ages, symptoms, neuroimaging findings, and treatments.
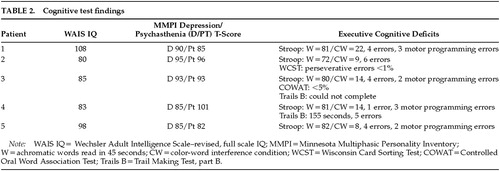
Each patient underwent a complete neuropsychological test battery including assessment of intellectual, memory, language, and visuoperceptual abilities and executive cognitive functions. Although it has been noted that there may be poor specificity for neuropsychological tests of “frontal” dysfunction,14 our cases are notable for the absence of prominent cognitive deficits except on tasks sensitive to executive cognitive functions (Trails B, Stroop color-word interference test, bimanual motor programming/response inhibition, verbal fluency), suggesting their deficits were focal in nature. The D and Pt (psychasthenia) scales on the MMPI-16815 provide psychometric measures of depression and OCD conditions, a T-score greater than 70 being considered “clinically significant.” Although our patients' elevated MMPI Pt scores could represent incidental preexisting character traits rather than acquired OCD pathology, it is notable that none of the patients had displayed obsessive-compulsive symptoms requiring treatment in their prior psychiatric care. The neurocognitive manifestations of executive cognitive deficits in our cases are summarized in Table 2.
CASE HISTORY/CLINICAL COURSE
Patient 1 is a 76-year-old married Caucasian woman who began treatment for depression when she was in her late 50s. After more than a decade of treatment for depression with tricyclic antidepressants, she developed OCD at age 72, with a prominent obsession that another woman might seduce her husband. The obsession was ego dystonic, but she nevertheless acted on a compulsion to continually monitor his location. At hospitalization, an MRI of the brain showed lacunar infarcts in the basal ganglia bilaterally. The etiology of the lesions is not known; there is nothing in her medical history that points to a causative factor. Her OCD was treated successfully with fluoxetine 40 mg qd, and her depressive symptoms subsequently resolved as well.
Patient 2 is a 61-year-old divorced African-American woman who developed OCD in her late 50s. She became obsessed with the thought that her neighbor might set her house on fire. She began counting drawers and cabinets in her kitchen many times per day and combing her hair repetitively, despite being sufficiently groomed. The thoughts were ego dystonic, and she became very anxious if she did not perform the counting and combing behaviors. She was misdiagnosed in a community clinic as having a psychotic disorder and treated with a typical antipsychotic. Her OCD did not improve, and she developed tardive dyskinesia (TD).
As her mood became more depressed, she was admitted to the hospital. Our evaluation included an MRI of the brain, which revealed bilateral basal ganglia infarcts. Borderline hypertension was noted in her past medical history at admission, and this may have been an etiological factor for the infarctions. She was diagnosed with OCD and major depression. We discontinued her antipsychotic treatment and placed her on clomipramine 225 mg qd, clonazepam 1.75 mg qd, and vitamin E 400 IU tid for TD. Her TD has resolved partially over 6 months.
Patient 3 is a 77-year-old married Caucasian woman who was admitted to our hospital service with ego-dystonic obsessions of a sexual nature regarding God, as well as obsessions that she would inadvertently harm someone in public and that she might kill her husband. Before coming to our attention she was treated for 10 years in another clinic for a psychotic disorder. She did not have psychiatric symptoms necessitating treatment prior to age 67. At that time she began treatment with haloperidol and then, after developing parkinsonism, was switched to risperidone. Upon admission to our service she had tongue fasciculations, bilateral upper and lower extremity tremors, head tremors, and truncal movements. Her Abnormal Involuntary Movements Scale score was 20. An MRI of the brain showed a right basal ganglia infarction. We discontinued risperidone and began treatment with fluoxetine for OCD and amantadine and vitamin E for parkinsonism. Both the OCD and the parkinsonism improved prior to discharge.
Patient 4 is a 70-year-old married Caucasian woman who had suffered from depression for almost 25 years. She had had five prior psychiatric hospitalizations since 1975 and was treated with maprotiline. Over the past several years she had begun developing OCD symptoms. She would ask her husband for reassurance in performing daily routine tasks, check the mirror for facial abnormalities, and organize her bathroom, all on a repetitive basis. Clinically, her depression always exacerbated her OCD. For approximately 2 years she has been treated with ECT and an outpatient combination of maprotiline 200 mg qhs and sertraline 50 mg qd. A CT of the head obtained at her last admission revealed bilateral basal ganglia calcifications. The etiology of these lesions is unknown, and there is nothing in her history to indicate a causative factor. At discharge, following another course of ECT, she was started on sertraline 100 mg qd and had remissions of both OCD and depression.
Patient 5 is a 77-year-old widowed Caucasian woman admitted to our service for the treatment of OCBs that developed precipitously about 2 years prior to admission. From 1988, following her husband's death, until the onset of OCBs, she was stabilized with pharmacotherapy. The OCBs developed after undergoing routine dental work. She developed an obsession that her teeth remained diseased and spent an inordinate amount of time seeking dental evaluations from various dentists. Her ego-syntonic obsessions and compulsions were treated initially with paroxetine, with temporary improvement. The symptoms returned and were followed by a decline in her mood and functioning. Several other outpatient treatments were attempted but failed, including venlafaxine, fluvoxamine, and lithium augmentation.
At admission, an MRI of the brain revealed ischemic changes in the head of the right caudate, the right lateral frontal convexity, and the left lateral parietal convexity. The etiology of these lesions is unknown. She underwent ECT with a course of six uncomplicated treatments. After the treatments her mood returned to premorbid baseline and she no longer mentioned having problems with her teeth.
It is notable that the use of medications with both antidepressant and OCD-reducing effects or the aggressive treatment of medication-refractory depression with ECT resulted in concomitant improvement of the patients' fixed obsessions and their depression. Treatment was more complicated in patients #2 and #3, who developed parkinsonism after they were previously treated with neuroleptics for what were regarded as psychotic delusions. Vitamin E and amantadine attenuated their movement disorders to some extent.
DISCUSSION
These cases support the view that if depressive symptoms are correlated with dysfunction of cortical structures, basal ganglia lesions will further predispose depressed patients to the development of obsessive-compulsive behaviors (and the obverse would hold as well). Models for the neuroanatomical circuitry of OCD have been proposed. Based on data from neuroimaging studies8–11 and case reports,2,3 these models implicate neuronal pathways on a CBGCT axis.4–6 A neuronal loop runs sequentially from the frontal cortex to the basal ganglia structures, which sends neuronal fibers to the medial thalamic nuclei. The loop is completed as fibers run from the thalamus back to the cortices.4 There is a convergence of neurons along this pathway, from dispersion in the cortical structures (orbitofrontal and cingulate) to restriction in basal ganglia structures and then further restriction into finely bundled neuronal fibers that go to thalamic nuclei. As a focus of pathology, the basal ganglia has an increased burden for the promotion of psychiatric symptoms because of the convergence phenomenon.
The lesions found in the basal ganglia in our patients could account for their OCD behaviors if this pathway is dysregulated. Functional studies that correlate with this anatomical postulate show increases in metabolic activity in the basal ganglia of OCD patients, and these patients also have significantly elevated metabolic activity in ipsilateral orbitofrontal cortices.16 A putative mechanism of overcompensation in residual basal ganglia structures of a lesioned basal ganglia likely accounts for some increased activity in this area of the brain. This increased activity in turn reduces inhibition at the thalamus, according to a model proposed by Insel,6 and the thalamus signals back to the cortex.
Our study is limited in several respects. All five patients are female, and four of the five are geriatric. This gives us a narrowed population sample. In addition, we do not know the etiologies of four of five of the lesioned basal ganglia, and we can only speculate on causes for Patient 2.
Although more research is needed to definitively elucidate underlying neuropathological mechanisms responsible for the psychiatric presentations of our patients, our case studies are consistent with the body of knowledge developed on basal ganglia disorders. First, they fit the neuroanatomical model of OCD that encompasses a cortical–basal ganglia–thalamic–cortical axis. Second, they demonstrate that the resolution of depressive and concurrent OCD symptoms can be interdependent in depression-prone patients who develop basal ganglia lesions, given that the CBGTC circuitry predisposes such patients to comorbidity. Finally, they illustrate the need for careful workup of older patients with recently acquired OCD because their symptoms may be mistaken for delusional disorders, placing them at risk for misdiagnosis and neuroleptic-induced extrapyramidal disorders.
 |
 |
1 Jenicke MA: Obsessive-compulsive disorder, in Comprehensive Textbook of Psychiatry, 6th edition, edited by Kaplan HI, Sadock BJ. Baltimore, Williams and Wilkins, 1995, pp 1218–1219Google Scholar
2 Kant R, Smith-Seemiller L, Duffy JD: Obsessive-compulsive disorder after closed head injury: review of literature and report of four cases. Brain Inj 1996; 10:55–63Crossref, Medline, Google Scholar
3 Laplane D, Levasseur M, Pillon B, et al: Obsessive-compulsive and other behavioral changes with bilateral basal ganglia lesions. Brain 1989; 112:699–725Crossref, Medline, Google Scholar
4 Modell JG, Mountz JM, Curtis GC, et al: Neurophysiologic dysfunction in basal ganglia/limbic striatal and thalamocortical circuits as a pathogenetic mechanism of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci 1989; 1:27–36Link, Google Scholar
5 Cummings JL: Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50:873–880Crossref, Medline, Google Scholar
6 Insel TR: Toward a neuroanatomy of obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49:739–744Crossref, Medline, Google Scholar
7 Robinson D, Wu H, Munne RA, et al: Reduced caudate nucleus volume in obsessive compulsive disorder. Arch Gen Psychiatry 1995; 52:393–398Crossref, Medline, Google Scholar
8 Rauch SL, Savage CR: Neuroimaging and neuropsychology of the striatum. Psychiatr Clin North Am 1997; 20:741–767Crossref, Medline, Google Scholar
9 Baxter LR, Schwartz JM, Bergman KS, et al: Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49:681–689Crossref, Medline, Google Scholar
10 Rauch SL, Jenicke MA, Alpert NM, et al: Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry 1994; 51:62–70Crossref, Medline, Google Scholar
11 Baxter LR, Phelps JM, Mazziotta JC, et al: Local cerebral glucose metabolic rates in obsessive-compulsive disorder: a comparison with rates in unipolar depression and normal controls. Arch Gen Psychiatry 1987; 44:211–218Crossref, Medline, Google Scholar
12 Hollander E, Cohen L, Richards M, et al: A pilot study of the neuropsychology of obsessive-compulsive disorder and Parkinson's disease: basal ganglia disorders. J Neuropsychiatry Clin Neurosci 1993; 5:104–107Link, Google Scholar
13 Head D, Bolton D, Hymans N: Deficit in cognitive shifting ability in patients with obsessive-compulsive disorder. Biol Psychiatry 1989; 25:929–937Crossref, Medline, Google Scholar
14 Reitan RM, Wolfson D: A selective and critical review of neuropsychological deficits and the frontal lobes. Neuropsychol Rev 1994; 4:161–198Crossref, Medline, Google Scholar
15 Overall JE, Higgins W, DeSchweintz A: Comparison of differential diagnostic discrimination for abbreviated and standard MMPI. J Clin Psychol 1976; 32:237–245Crossref, Medline, Google Scholar
16 Baxter LR: Neuroimaging studies of obsessive-compulsive disorder. Psychiatr Clin North Am 1992; 15:871–884Crossref, Medline, Google Scholar



