Schizophrenia Subgroups Differing in Dichotic Listening Laterality Also Differ in Neurometabolism and Symptomatology
Abstract
Schizophrenia patients vary in right ear advantage (REA) on dichotic listening tests for assessing left hemispheric dominance for language processing. The authors examined if patients with low REA differed from other patients in symptoms and in resting brain metabolism. SPECT was conducted during visual fixation for 9 healthy control subjects and 16 schizophrenia patients: 8 with normal and 8 with diminished REA. REA-diminished patients had greater positive symptoms and lower mental status scores (all P<0.05) and had right middle temporal gyrus hypermetabolism. Both schizophrenia groups had decreased right frontal and increased medial temporal lobe metabolism vs. control subjects. REA-diminished patients had right temporal lobe hypermetabolism under a resting condition (eyes open, visual fixation). Results suggest reduced right ear (left hemisphere) advantage for dichotic word perception in schizophrenia is related to a predisposition to overactivate right temporal lobe regions and to positive symptoms. In contrast, the prefrontal–medial temporal imbalance present in both patient groups may typify the schizophrenia syndrome.
Schizophrenia patients show variance in many biological dimensions, including brain hemispheric advantage for language processing. This variable can be indexed noninvasively by using dichotic listening tests, in which a different word or syllable is simultaneously presented to the two ears. These tests generally show a right ear advantage (REA) of 10% to 15% in right-handed healthy adults, which reflects the superiority of the left hemisphere for processing language-related stimuli. The Fused Rhymed Words Test is one of the most reliable and valid dichotic listening tests.1,2 In this test, the different words presented to the two ears fuse to form a single percept and the subject reports the word that was heard. It involves minimal memory demand and is less subject to lateralized attentional effects than other dichotic listening tests. Most studies using this test or similar dichotic syllable tests have found reduced or absent right ear (left hemisphere) advantage in schizophrenic patients.3–9 There are, however, marked individual differences among patients, with many showing a normal REA. This variability in perceptual asymmetry among schizophrenia patients has been related to symptom differences; for example, REA for dichotic words or syllables was more evident in patients with positive symptoms, in particular auditory hallucinations.3,10,11
Studies using dichotic listening as an imaging neuroactivation task have shown the increases in left temporal lobe regional cerebral blood flow (rCBF) that might be expected to accompany right ear advantages for language stimuli.12 Schizophrenia patients in another dichotic study (with instructions to attend to their right or their left ear) had high left superior temporal gyrus rCBF in all conditions, unlike control subjects.13 Dichotic listening ear advantages and accompanying rCBF changes may be related to the neural systems that integrate auditory processing between the hemispheres, as has been proposed,13 or they may reflect a task-independent neural predisposition to activate one hemisphere over the other. Individual differences in perceptual asymmetry may be due to stable differences in hemispheric activity.14 For example, asymmetries of brain electrical activity obtained at rest (alpha power) were related to hemispheric advantage for dichotic listening performance;15 that is, healthy adults who had greater left-sided temporoparietal activation during resting EEG showed a larger right ear advantage for dichotic syllables.
These findings raise the possibility that the reduced REA in schizophrenic patients might reflect a characteristic activational asymmetry pattern that favors right over left temporoparietal activation. Support for this hypothesis comes from a functional magnetic resonance imaging study,16 which found evidence that schizophrenia patients showed reduced left and increased right temporal activation during speech perception. It was proposed that this activational asymmetry resulted from an underlying asymmetry of the surface area of the planum temporale, a structure on the posterior surface of the superior temporal gyrus that provides an anatomical basis for left hemisphere superiority for language processing. Reversal of the normal asymmetry of the planum temporale in schizophrenia is also described, with a markedly larger area on the right side and a somewhat smaller area on the left side.17
The present study examined rCBF of schizophrenic patients who were grouped by their perceptual asymmetry on the Fused Rhymed Words Test. We hypothesized that if reduced right ear advantage in schizophrenia for perceiving dichotic words reflects a stable, activational asymmetry in these patients, then it should also be evident under resting conditions. The main question addressed in this study is whether schizophrenic patients with little or no REA for dichotic words would show evidence of reduced left temporal activation, enhanced right temporal activation, or both when compared with healthy control subjects. A secondary purpose was to examine if this schizophrenia subgroup with reduced REA would differ from patients with normal perceptual asymmetry, both in their rCBF at rest and on measures of positive symptoms.
METHODS
Subjects
A sample of 9 healthy comparison subjects and 16 patients with DSM-III-R diagnoses of schizophrenia from an inpatient schizophrenia research unit underwent single-photon emission computed tomography (SPECT) imaging. After complete description of the study to the subjects, written consent was obtained for this Institutional Review Board–approved study. They were physically healthy, as indicated by recent physical examinations, laboratory evaluation (SMA20), complete blood count, urinalysis, and thyroid function tests. All subjects were right-handed, had no recent substance use history, and had no history of neurological illness. The subjects had been screened for auditory acuity, and no subject had a hearing loss greater than 30 dB in either ear or an ear difference greater than 10 dB at 500, 1,000 and 2,000 Hz.
The patients were assigned to one of two groups based on their laterality scores on the Fused Rhymed Words Test. The number of right (R) or left (L) ear words correctly reported was used to compute a standard laterality quotient, as [(R−L)/(R+L)]×100. The cutoff laterality quotient used for dividing patients into subgroups was 10%, which was based on the average laterality quotient for 62 healthy adults who were tested in our laboratory on the Fused Rhymed Words Test.18 Eight patients who had an REA equal to or greater than 10% were matched by sex, age (within 5 years), and type of neuroleptic treatment to patients who had less than 10% or no REA. This yielded a subgroup of patients with a large right ear (left hemisphere) advantage having a mean laterality quotient of 25.8% (SD=17.1; range 10.0 to 63.0) and a subgroup with little or no REA having a mean laterality quotient of 2.8% (SD=3.8; range –4.4 to 8.1). All patients had been on stable doses of neuroleptic (or were neuroleptic-free) for a minimum of 4 weeks prior to the imaging. Two patients in each group were neuroleptic-free and the rest were taking atypical antipsychotic medications; 4 in each group were taking clozapine, 1 patient in each group was taking risperidone, and 1 in each group was taking olanzapine.
Diagnoses and Symptoms
Structured clinical interviews were conducted with the Diagnostic Interview for Genetic Studies19 by one of three clinicians (two with master's degrees and one with a Ph.D. in clinical psychology). The interviewers completed initial calibration checks showing high interrater reliability (i.e., kappa>0.8 for individual symptom ratings and 95% agreement on diagnosis). DSM-III-R diagnoses were made using data from the structured interviews, past records, and symptom ratings, and represented a consensus among clinical and research staff. Comparison subjects were clinically interviewed and had no personal or family history of psychosis and no Axis I diagnosis within the past 2 years. Patient data included age, sex, education, age at onset of positive symptoms, and Mini-Mental State Examination scores. Symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS), the Brief Psychiatric Rating Scale (BPRS), and the Hamilton Rating Scale for Depression.
Dichotic Listening
The Fused Rhymed Words Test has been described in detail elsewhere.5 Briefly, single-syllable word pairs differing only in the initial consonant were presented simultaneously in the right and left ear (e.g., coat, goat), via a matched pair of TDH-49 headphones, at a comfortable level of 75 dB SPL. The words fuse into a single percept, and the subjects use a multiple-choice answer sheet to indicate the word they heard. Four 30-item blocks yield 120 trials.
Imaging
SPECT imaging was done by using 20 mCi of technetium-99m d,l-hexamethylpropyleneamine oxime (Ceretec; Amersham, Arlington Heights, IL) during a resting condition of eyes open with visual fixation. The isotope was injected through an intravenous line in the left arm 3 minutes after the subjects had commenced visual fixation on the intersection of a blue crosshair design (10 inches by 6 inches). Research staff monitored the subjects during the 15-minute imaging condition to ensure their compliance with the instructions. The subjects then sat quietly for an additional 20 minutes before undergoing a 20-minute SPECT scan on a rotating triple-head Picker-PRISM 3000 camera with a Leuhr high-resolution fan beam collimator (Picker International, Cleveland, OH). During the task, subjects had head immobilization to avoid motion.
Projection image data were acquired in a continuous acquisition mode at 7.5-second intervals, using 128×128 matrix for a total of 120 images over a 360-degree rotation orbit. Total scan time was 20 minutes, during which four 5-minute rapid acquisition sequence image data sets were acquired. Images were reconstructed by filtered back-projection, after processing with a 5th-order Butterworth filter with a cutoff frequency of 0.3–0.4 cycles/second, and subsequent attenuation correction using a 0.110 factor. Images were reconstructed in the transaxial plane, parallel to the orbitomedial line. The head angle was set at the time of acquisition by aligning external landmarks on the subject's head with a laser beam.
Image Analysis
The transaxial reconstructed images of 3.67 mm thickness per pixel were then transmitted to a Silicon Graphics workstation. The raw image files were read into an automated 3D image analysis software package (Sensor; MedX, Ocala, FL) and subsequently saved in an ANALYZE file format. These files were then imported into the SPM95 (Statistical Parametric Mapping) software package.20 The transformation of SPECT scans into the Talairach coordinate system was performed by using the “Normalization” function of the SPM95 software. This function employed the technique of minimizing the sum of squares between each of our images and the reference positron emission tomography (PET) image that was provided by the software and already transformed into Talairach space. Thus, all of the scans were normalized to the Talairach and Tournoux coordinate system.21 The scans were then smoothed by using a Gaussian filter with a kernel of 12 mm full width half maximum. Global differences were factored out as a covariate, voxel by voxel, using the analysis of covariance model described by Friston et al.20 Areas of increase and decrease in rCBF in schizophrenia groups compared with control subjects and compared with one another were identified for a Z-value threshold of 2.33, P=0.01, corrected for multiple comparisons with P=0.05. Significant rCBF changes were displayed in SPM95 as statistical parametric maps in Talairach space, both as the probabilities of cluster size and as activation magnitudes based on Z-scores. Two contrasts were separately examined to evaluate rCBF increases and decreases in parallel for each of the patient groups. Schizophrenia subgroups were contrasted with normal subjects. The results were superimposed on a Talairach-registered T1-weighted magnetic resonance image (MRI) provided by SPM95 software.
RESULTS
Clinical Data
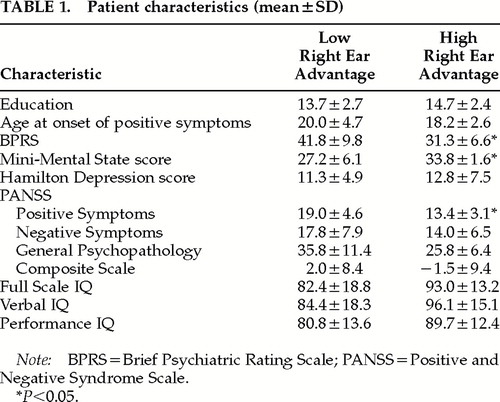
Each patient subgroup had 6 male and 2 female patients; the control group included 6 males and 3 females. Patient groups with higher and lower REA did not differ from one another in mean age (25.9±6.9 vs. 29.8±5.9 years; means±SD reported), or from the control group's mean age (30.0±6.7 years). At the time of imaging, the patient group with diminished REA had significantly higher PANSS positive symptom and BPRS scores and lower Mini-Mental State Examination scores (Table 1).
Imaging Data
The imaging data for the schizophrenia groups with higher (normal) and lower (diminished) REA on dichotic listening were first contrasted with those of the healthy comparison group for regions of significantly increased and decreased rCBF.
The schizophrenia group with normal high REA (Figure 1) had decreased rCBF in a right-sided 462-voxel cluster (P=0.110), centered at {x, y, z=50, 14, 8}, that included the middle and inferior frontal gyri, precentral gyri, and insula. There were two regions of increased rCBF: a 178-voxel region (P=0.641) centered at {x, y, z=30, –26, –24} and a 1,152-voxel region (P= 0.002) centered at {x, y, z= –28, –42, –24}. These clusters included the bilateral parahippocampus, left fusiform gyrus, and bilateral cerebellum (posterior and anterior).
The schizophrenia group with diminished REA (Figure 2) had regions of decreased rCBF that were nearly identical to those of control subjects, as did the group with higher REA described above. These hypometabolic regions were in a single right-sided 830-voxel region (P=0.013) centered at {x, y, z=50, 14, 4} and including the middle and inferior frontal gyri, precentral gyri, and insula. This patient group showed three clusters of significantly increased rCBF, compared with the control subjects. These regions included the identical hypermetabolic regions seen in the normal-REA schizophrenia patients described above, and an area of increased rCBF unique to this subgroup. The regions of increased rCBF in common with the other patient subgroup were bilateral parahippocampal gyri, fusiform gyrus, cerebellum, medial frontal gyrus, paracentral lobule, and postcentral gyrus.
A hypermetabolic region in the right-sided middle temporal and inferior temporal gyri was unique to the schizophrenia group with diminished REA. It extended into the right uncus, amygdala, substantia nigra, thalamus, and pons. Talairach-registered T1-weighted MRI showed these clusters to be as follows: 1,010 voxels (P=0.005) centered at {x, y, z=6, –22, –24}; 1,575 voxels (P<0.001) centered at {x, y, z=4, –38, 60}; and 627 voxels (P= 0.041) centered at {x, y, z= –28, –42, –24}.
When the schizophrenia groups were compared with each other, the diminished-REA group was hypermetabolic in the right middle temporal gyri, as well as in bilateral precentral and postcentral gyri. This group had significantly lower rCBF than the normal-REA group in the right insula and right inferior frontal gyrus.
DISCUSSION
We found that a group of schizophrenia patients who lacked the normal left hemispheric superiority for language on dichotic listening had increased right middle temporal lobe metabolism, compared with both the healthy control subjects and the schizophrenia patients with normal dichotic asymmetry during a resting state. This finding suggests that these patients with diminished REA have a relatively stable predisposition to overactivate the right relative to the left hemisphere, and that their abnormal brain asymmetry is not restricted to auditory processing. These patients also had greater positive symptoms, higher BPRS scores, and lower Mini-Mental State scores than the schizophrenia patients who had normal (high) REA. The two schizophrenia subgroups, however, showed nearly identical regions of prefrontal hypoperfusion and medial temporal lobe hyperperfusion compared with the healthy control subjects.
The finding of greater positive symptoms in schizophrenic patients with diminished REA is consistent with prior findings for the Fused Rhymed Words Test3 and with studies finding an association between hallucinations and reduced REA for dichotic consonant-vowel syllable tests.10,11 Also, the tendency of schizophrenic patients to preferentially rotate toward their left side when circling has been correlated with delusions.22–25 Metabolic asymmetries have been previously described in schizophrenia, with greater right than left temporal lobe rCBF being reported26 even in neuroleptic-naive patients.27
Brain hemispheric imbalances have also been related to positive symptoms. Increased right hemisphere rCBF28,29 and reduced left temporal lobe volumes30,31 have been associated with hallucinations and thought disorder. In contrast, Gruzelier et al.32 have reviewed neuropsychologic and electrophysiologic evidence for a hemispheric imbalance model, in which an “active syndrome” (including delusions, overactivity, and hallucinations) was associated with left hemisphere integrity and loss of right hemisphere function. Their “active” versus “withdrawn” distinction is not, however, directly comparable with the positive versus negative symptom dichotomy. There may also be differences in the diagnostic subtypes of the schizophrenic patients that could contribute to the conflicting findings across studies. For instance, patients having paranoid schizophrenia have been reported to have a stronger REA than nonparanoid patients on dichotic recall tests.33 There is a need for further study of the clinical correlates of hemispheric asymmetries in schizophrenia, using more direct electrophysiologic or neuroimaging measures during the performance of dichotic or other laterality tests.
A question may be raised as to whether the differences in dichotic listening or brain metabolism could be due to the effects of the antipsychotic medications taken by most of the patients. The subgroup of patients with diminished REA were on essentially the same medications as the patients with normal REA; thus, differences between these groups in either dichotic listening or metabolism are not likely to be due to the medications. Also, in our prior study using the Rhymed Fused Word Test, we did not find a significant difference in REA of patients who were tested both on and off antipsychotics.3 Although antipsychotics were found in some studies to normalize left hemisphere performance at the expense of right hemisphere performance,34 these studies used dichotic recall and hemispatial attention tests that assess different aspects of lateralized brain function than does the Rhymed Fused Word Test used in the present study. Moreover, antipsychotics would, if anything, act to normalize hemispheric asymmetries, which could not account for the right temporal hypermetabolism in the subgroup with diminished REA.
Questions might also arise as to whether the difference in REA and metabolism between the patient subgroups could be due to a difference in the overall cognitive deficits of patients in these groups. The subgroup of patients with diminished REA also differed from the subgroup with normal REA in having higher BPRS scores and lower Mini-Mental State scores. Although we cannot dismiss the possibility that these differences affected dichotic listening test results, we have not generally found that patients with greater overall clinical severity have smaller REA on the Rhymed Fused Words Test. One of the advantages of this test is that the paired dichotic words fuse to form a single percept and patients only need to report the single word that they perceived. It is therefore less likely to be influenced by an overall cognitive deficit.
Differences in dichotic listening perceptual asymmetry may indicate underlying differences in the predisposition to activate one hemisphere over the other. Imaging subjects during a number of cognitive tasks, including dichotic listening, would be necessary to examine the breadth and stability of these metabolic asymmetries. The relative right temporal lobe metabolic dominance may explain why the left temporal lobe language regions are not preferentially activated during the perception of language stimuli. Alternatively, the experience of positive symptoms, like that of hallucinations, could be associated with enhanced right temporal lobe activity in schizophrenia. Although neither schizophrenia group showed left temporal lobe hypometabolism, we cannot rule out the possibility that right temporal hypermetabolism was in some way compensatory for a left hemispheric deficit. We also cannot be certain whether a region of increased metabolic activity reflects an increase in excitatory or inhibitory neural control.
The decreased prefrontal perfusion and increased medial temporal perfusion seen in both schizophrenia groups may reflect a trait neurometabolic abnormality that is related to the schizophrenia syndrome per se. We have previously reported this perfusion pattern for medication-free schizophrenia patients for a resting visual fixation task.35 A theory of imbalance or disconnection between the frontal cortex and the medial temporal lobe has been proposed to account for schizophrenia,36 and such abnormalities have been demonstrated in other PET and SPECT studies.37 The right-sided cortical-subcortical hypermetabolism we found for the diminished-REA patients might normalize with clinical improvement. Although dichotic asymmetries do not appear to be related to neuroleptic medication status,33,38 they do vary with clinical state.5,39,40
Differences in ear advantage may reflect overall cognitive deficits that are also reflected in the metabolism findings. Whether the rCBF differences between these schizophrenia subgroups reflect clinical state differences or stable structural or other trait-dependent differences remains to be determined. Retesting the patients in different clinical states could help address this issue.
ACKNOWLEDGMENTS
This work was supported by the Mathers Foundation (D.M.), by a National Institute of Mental Health Developing Schizophrenia Research Center Grant and by Grants 5P20 MH50727 and MH50715 (G.B.).
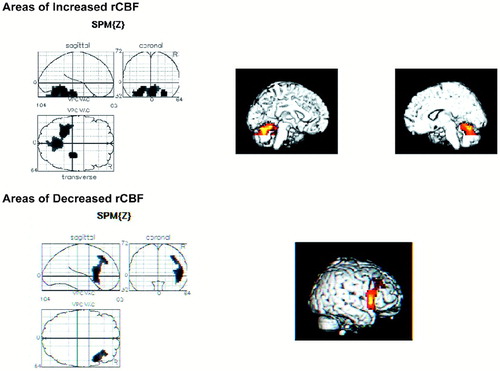
FIGURE 1. Regional cerebral blood flow for patients with normal REA on dichotic listening vscontrol subjects (see Results section in text for full description).
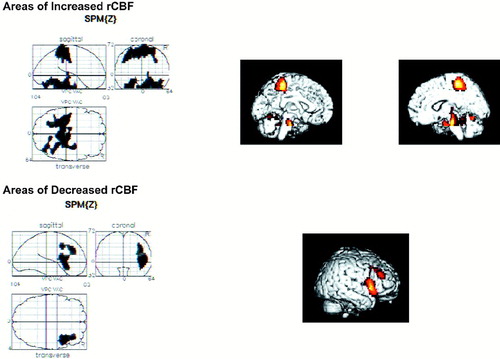
FIGURE 2. Regional cerebral blood flow for patients with diminished REA on dichotic listening vscontrol subjects (see Results section in text for full description).
 |
1 Wexler BE, Halwes T: Increasing the power of dichotic methods: the Fused Rhymed Words Test. Neuropsychologia 1983; 21:59–66Crossref, Medline, Google Scholar
2 Zatorre RJ: Perceptual asymmetry on the dichotic fused words test and cerebral speech lateralization determined by the carotid sodium amytal test. Neuropsychologia 1989; 27:1207–1219Google Scholar
3 Bruder G, Rabinowicz E, Towey J, et al: Smaller right ear (left hemisphere) advantage for dichotic fused words in patients with schizophrenia. Am J Psychiatry 1995; 152:932–935Crossref, Medline, Google Scholar
4 Lishman WA, Toone BK, Colbourn CJ, et al: Dichotic listening in psychotic patients. Br J Psychiatry 1978; 132:333–341Crossref, Medline, Google Scholar
5 Wexler BE, Giller EL Jr, Southwick S: Cerebral laterality, symptoms, and diagnosis in psychotic patients. Biol Psychiatry 1991; 29:103–116Crossref, Medline, Google Scholar
6 Raine A, Andrews H, Sheard C, et al: Interhemispheric transfer in schizophrenics, depressives, and normals with schizoid tendencies. J Abnorm Psychol 1981; 98:35–41Crossref, Google Scholar
7 Wale J, Carr V: Dichotic listening asymmetries and psychotic symptoms in schizophrenia. Psychiatry Res 1988; 25:31–39Crossref, Medline, Google Scholar
8 Bruder G, Kayser J, Tenke C, et al: Left temporal lobe dysfunction in schizophrenia: event-related potential and behavioral evidence from phonetic and tonal dichotic listening tasks. Arch Gen Psychiatry 1999; 56:267–276Crossref, Medline, Google Scholar
9 Loberg EM, Hugdahl K, Green MF: Hemispheric asymmetry in schizophrenia: a “dual deficits” model. Biol Psychiatry 1999; 45:76–81Crossref, Medline, Google Scholar
10 Levitan C, Ward PB, Catts SV: Superior temporal gyral volumes and laterality correlates of auditory hallucinations in schizophrenia Biol Psychiatry 1999; 1:46:955–962Google Scholar
11 Green MF, Hugdahl K, Mitchell S: Dichotic listening during auditory hallucinations in patients with schizophrenia. Am J Psychiatry 1994; 151:357–362Crossref, Medline, Google Scholar
12 Hugdahl K, Bronnick K, Kyllingsbaek S, et al: Brain activation during dichotic presentations of consonant-vowel and musical instrument stimuli: a 15O-PET study. Neuropsychologia 1999; 37:431–440Crossref, Medline, Google Scholar
13 O'Leary DS, Andreasen NC, Hurtig RR, et al: Auditory attentional deficits in patients with schizophrenia: a positron emission tomography study. Arch Gen Psychiatry 1996; 53:633–641Crossref, Medline, Google Scholar
14 Levy J, Heller W, Banich MT, et al: Are variations among right-handed individuals in perceptual asymmetries caused by characteristic arousal differences between hemispheres? J Exp Psychol Hum Percept Perform 1983; 9:329–359Google Scholar
15 Davidson RJ, Hugdahl K: Baseline asymmetries in brain electrical activity predict dichotic listening performance. Neuropsychology 1996; 10:241–246Crossref, Google Scholar
16 Woodruff PW, Wright IC, Bullmore ET, et al: Auditory hallucinations and the temporal cortical response to speech in schizophrenia: a functional magnetic resonance imaging study. Am J Psychiatry 1997; 154:1676–1682Google Scholar
17 Barta PE, Pearlson GD, Powers RE, et al: Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Google Scholar
18 Malaspina D, Friedman JH, Kaufmann C, et al: Psychobiological heterogeneity of familial and sporadic schizophrenia. Biol Psychiatry 1998; 43:489–496Crossref, Medline, Google Scholar
19 Nurnberger C, York C, Kaufmann C, et al: Diagnostic interview for genetic studies. Arch Gen Psychiatry 1994; 51:849–859Crossref, Medline, Google Scholar
20 Friston KJ, Holmes AP, Worsley KJ, et al: Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping 1995; 2:189–210Crossref, Google Scholar
21 Talairach J, Tournoux P: A Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
22 Bracha HS: Asymmetric rotational (circling) behavior, a dopamine-related asymmetry: preliminary findings in unmedicated and never-medicated schizophrenic patients. Biol Psychiatry. 1987; 22:995–1003Google Scholar
23 Bracha HS, Livingston RL, Clothier J, et al: Correlation of severity of psychiatric patients' delusions with right hemispatial inattention (left-turning behavior). Am J Psychiatry 1993; 150:330–332Crossref, Medline, Google Scholar
24 Lyon N, Satz P, Fleming K, et al: Left turning (swivel) in manic patients. Schizophr Res 1992; 7:71–76Crossref, Medline, Google Scholar
25 Lyon N, Satz P: Left turning (swivel) in medicated chronic schizophrenic patients. Schizophr Res 1991; 4:53–58Crossref, Medline, Google Scholar
26 Russell JM, Early TS, Patterson JC, et al: Temporal lobe perfusion asymmetries in schizophrenia. J Nucl Med 1997; 38:607–612Medline, Google Scholar
27 Andreasen NC, O'Leary DS, Flaum M, et al: Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet 1997; 349:1730–1734Google Scholar
28 Cleghorn JM, Garnett ES, Nahmias C, et al: Increased frontal and reduced parietal glucose metabolism in acute untreated schizophrenia. Psychiatry Res 1989; 28:119–133Crossref, Medline, Google Scholar
29 Volkow ND, Wolf AP, Van Gelder P, et al: Phenomenological correlates of metabolic activity in 18 patients with chronic schizophrenia. Am J Psychiatry 1987; 144:151–158Crossref, Medline, Google Scholar
30 Barta PE, Pearlson GD, Powers RE, et al: Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Google Scholar
31 Shenton ME, Kikinis R, McCarley RW, et al: Application of automated MRI volumetric measurement techniques to the ventricular system in schizophrenics and normal controls. Schizophr Res 1991; 5:103–113Crossref, Medline, Google Scholar
32 Gruzelier J, Seymour K, Wilson L, et al: Impairments on neuropsychologic tests of temporohippocampal and frontohippocampal functions and word fluency in remitting schizophrenia and affective disorders. Arch Gen Psychiatry 1988; 45:623–629Crossref, Medline, Google Scholar
33 Gruzelier JH, Hammond NV: Lateralized deficits and drug influences on the dichotic listening of schizophrenic patients. Biol Psychiatry 1980; 15:759–779Medline, Google Scholar
34 Tomer R, Flor-Henry P: Neuroleptics reverse attention asymmetries in schizophrenic patients. Biol Psychiatry 1989; 25:852–860Crossref, Medline, Google Scholar
35 Malaspina D, Storer S, Furman V, et al: SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry 1999; 46:89–93Crossref, Medline, Google Scholar
36 Friston KJ, Frith CD, Fletcher P: Abnormal fronto-temporal interactions in schizophrenia, in Biology of Schizophrenia and Affective Disorders, edited by Watson SJ. New York, Raven, 1994, pp 421–450Google Scholar
37 Fletcher P, McKenna PJ, Fristo KJ, et al: Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage 1999; 9:337–342Crossref, Medline, Google Scholar
38 Bruder GE: Cerebral laterality and psychopathology: perceptual and event-related potential asymmetries in affective and schizophrenic disorders, in Brain Asymmetry, edited by Davidson RJ, Hugdahl K. Cambridge, MA, MIT Press, 1995, pp 661–691Google Scholar
39 Johnson O, Crockett D: Changes in perceptual asymmetries with clinical improvement of depression and schizophrenia. J Abnorm Psychol 1982; 91:45–54Crossref, Medline, Google Scholar
40 Wexler BE, Heninger GR: Alterations in cerebral laterality during acute psychotic illness. Arch Gen Psychiatry 1979; 36:278–284Crossref, Medline, Google Scholar



