A Volumetric Study of Hippocampus and Amygdala in Depressed Patients With Subjective Memory Problems
Abstract
MRI was used to measure amygdalar and hippocampal volumes in 14 nondemented depressed patients who persistently complained of “memory” difficulties and in 14 control subjects. Mild neuropsychological impairment had been detected in 5 patients before the study but had later improved. The volume of the left amygdala was smaller in depressed subjects, and there was a trend for smaller left hippocampus in the 5 patients who had exhibited mild cognitive impairment. The authors conclude that subjective memory complaints in depressed patients do not translate into a clinical picture of dementia, but that abnormalities in the amygdala and hippocampus may be relevant in explaining affective and cognitive symptoms.
Cognitive abnormalities have been well documented in depression. These abnormalities may cut across a range of cognitive domains, but memory is usually affected.1,2 There is also increasing evidence that cognitive abnormalities may persist during euthymic phases.3,4 The neural networks responsible for the cognitive abnormalities of depression are not well understood, but reductions in amygdalar and hippocampal volume5–7 have been reported.
More than 20% of the general population complain of poor memory, increasing to more than 40% in those over the age of 65. Objective memory impairment is about half those rates, but the gap narrows with increasing age.8 Subjective memory problems are also common in depression.9 The mechanisms underlying these complaints and their relationship to the reported abnormalities in medial temporal structures remain to be determined.
We studied a group of depressed patients with persistent memory complaints in whom the presence of dementia had been excluded. We used MRI techniques to measure the volumes of hippocampus and amygdala to test whether there was a link between volumetric reduction, depression, and subjective memory difficulties.
METHODS
Subjects
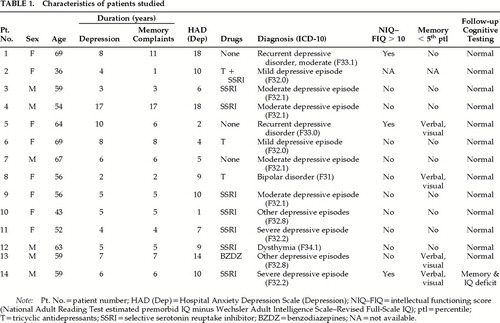
Fourteen nondemented patients (8 women) who fulfilled ICD-10 criteria for depressive illness (any type) and who persistently complained of “memory” difficulties were recruited from current outpatient attenders to the National Hospital for Neurology and Neurosurgery (NHNN). The characteristics of these patients are detailed in Table 1. The “memory” complaints included difficulty in learning new things, general forgetfulness, forgetting words, and lack of concentration. Patients were excluded if they fulfilled the DSM-IV criteria for dementia, scored less than 27 on the Mini-Mental State Examination (MMSE),10 or had a history of neurological disease, head injury leading to loss of consciousness, or alcohol or drug abuse. Seven patients were taking selective serotonin reuptake inhibitors, 2 tricyclic antidepressants, 1 benzodiazepines, and 1 a combination of these drugs. Five patients were excluded because they did not fulfill diagnostic criteria for depression (n=1), had probable Alzheimer's disease (n=2), or scored <27 on the MMSE (n=2).
Fourteen subjects (8 women) from a bank of healthy volunteers participating in other imaging studies were used as controls for MRI data. They were individually matched to the patients by gender and within 3 years for age. Control subjects were excluded if they had sustained a head injury leading to unconsciousness or if they had a history of neurological or psychiatric illness. None had subjective memory difficulties.
All subjects gave their written consent for the study.
Psychiatric Assessment
Patients were interviewed by one of us (A.v.G.) using a symptom checklist to reach an ICD-10 diagnosis. The Hospital Anxiety and Depression Scale (HAD)11 was used to measure depression and/or anxiety at the time of the interview. Patients were considered to be euthymic if their HAD score was below 9 (Table 1).
Neuropsychological Assessment
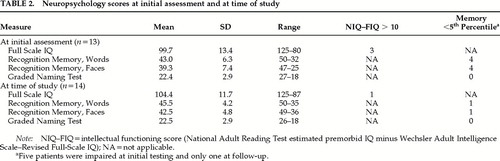
All patients except one (patient #2) had been previously assessed when first seen at the NHNN. For this study, a further assessment was performed in 1998 on the same day as the psychiatric assessment. The mean interval between the two assessments was 3.5 years (SD=1.9). The following tests were administered: a short version of the Wechsler Adult Intelligence Scale–Revised,12 consisting of four verbal subtests (Vocabulary, Arithmetic, Digit Span, and Similarities) and three performance subtests (Block Design, Picture Completion, and Picture Arrangement);12 the Recognition Memory Test for words and faces;13 and the Graded Naming Test.14 The National Adult Reading Test (NART)15 was administered to obtain a measure (reading IQ equivalent) of premorbid optimal level of intellectual functioning.
Three derived scores were calculated. The intellectual functioning score was the difference between the NART and the Full Scale IQ. A difference greater than 10 was taken as evidence of intellectual decline. The memory and naming scores were derived by converting the standardized test performances into percentile scores. Scores at or below the 5th percentile were taken to indicate memory or naming impairment.
Control subjects had been screened to exclude cognitive impairment prior to their inclusion in the bank of healthy volunteers. The following neuropsychological tests had been used for that purpose: the Mini-Mental State Examination (MMSE),10 the NART,15 the Recognition Memory Test,13 the Paired Associates Learning test,16 and Raven's Progressive Matrices.17 Their performance on these tests was within the normal range. Some of these tests were different from those given to the depressed subjects, and no comparisons were made between the scores of the two groups.
Magnetic Resonance Imaging
All scans were performed on a 1.5-tesla Signa system (GE Medical Systems, Milwaukee, WI, USA). The scans included routine sagittal T1-weighted and axial (5-mm thick) proton density and T2-weighted sequences. Volumetric imaging was performed by using a spoiled gradient echo technique with 24 cm field of view, yielding 124 contiguous 1.5-mm-thick coronal slices through the head on a 256*128 matrix. The acquisition parameters were TR=35, TE=5, NEX=1, FLIP=35.
Volumetric measurements were performed blind to subject identity and group membership. Images were randomly left-right flipped to ensure blindness to brain side. Digitized images were analyzed by using MIDAS software.18
Intracranial volume was measured as follows. The inner border of the skull was outlined on the T2-weighted scans by using a semiautomated thresholding method (approximately 10 axial slices). Intracranial volume below the cerebellum was excluded. There was a high intrarater reliability (Spearman's rho=1.0, P<0.0001).
The hippocampal formation included the dentate gyrus, the hippocampus proper, the subiculum, and the alveus.19 The region was first manually outlined in the sagittal view and then edited and checked in the coronal view. The superior and anterior borders were delineated by the outer limits of the alveus and the fimbria. The anterior disarticulation of the hippocampus from the amygdala was performed on the sagittal view. The inferior border was formed by the white matter of the subiculum. The wall of the lateral ventricle was taken as the lateral boundary, and the medial border corresponded to adjacent cistern. The posterior limit was arbitrarily defined as the coronal slice where the fornix was seen in its longest unbroken extent. Each region involved approximately 20 sagittal or 25 coronal slices in each hemisphere. After a 2- to 3-week interval, the selected hippocampal regions were rechecked for accuracy and minor adjustments were made, if necessary, in a blinded and randomized manner. Twelve hippocampi were measured twice to determine the intrarater reliability (Spearman's rho= 0.916. P<0.0001).
The amygdala was measured by a similar procedure. Approximately 20 sagittal and 15 coronal slices were processed. The superior limit corresponded to a line connecting the most inferior point of the lateral fissure with the most lateral part of the paramedian cistern and, more posteriorly, the superior and lateral border of the optic tract. The inferior, medial, and lateral borders were determined by the boundary between amygdala and adjacent white matter. The most rostral coronal slice was taken as the one where the temporal and frontal lobes were no longer separate. The posterior boundary was formed by the disarticulation with the hippocampus as described above. Fourteen amygdalae were measured twice to determine the intrarater reliability (Spearman's rho=0.917. P<0.0001).
Data Analysis
Hippocampus/intracranial and amygdala/intracranial ratios were calculated to control for brain size. Two- tailed nonparametric statistics (Mann-Whitney U-test) were used to compare volumetric ratios and paired t- tests to compare age, NART IQ, and MMSE between groups. Spearman's correlation coefficient was used to explore intrarater reliability and correlations between volumetric and other data.
RESULTS
Patients and control subjects were well matched for gender and age (patients: mean 57.6 years, range 37–69; controls: 58.1 years, range 36–72). There was a trend for the control subjects to have higher estimated premorbid IQ (patients: NART=105, SD=12.7; control subjects: NART=114.9, SD=9.1). The difference was not significant.
Two patients fulfilled ICD-10 criteria for recurrent depressive disorder, 10 for depressive episode, 1 for bipolar disorder, and 1 for dysthymia. Only 2 patients were considered to have been severely depressed; for the rest, depression was mild to moderate. For most patients, subjective memory difficulties appeared at the same time as the affective symptoms (Table 1). At the time of the study 6 patients were euthymic (HAD depression score <9).
The results from the initial assessment are given in Table 1. Eight patients had no cognitive impairment. Of the 5 remaining, 2 showed both intellectual and memory underfunctioning, 2 poor performance on memory tests, and 1 selective intellectual underfunctioning. All patients performed within normal limits on the naming test.
At the time of the study, the patients were reassessed with the same intelligence, memory, and naming tests. Individual summary results are shown in Table 1 and group mean scores in Table 2. The performance of the whole group had improved, and 13 patients obtained scores entirely within normal limits. Only 1 patient continued to have intellectual underfunctioning and poor performance on memory tests, but his performance on the easy-recognition memory test20 had improved (scores at first assessment: words 14/25, faces 16/25; at follow-up: words 21/25, faces 19/25). This patient had experienced severe depression, and he scored above the HAD cutoff point at follow-up.
Clinical Correlations
There were no significant age differences between the 5 patients (3 women and 2 men) with initial cognitive impairment and the rest. Three of the 5 were euthymic at follow-up, as were 4 of the unimpaired 9. The frequency of subjective “memory” problems at follow-up was much the same in the two groups (3 of the 5 initially impaired and 4 of the 9 unimpaired felt their memory was better). Five of the 7 patients with subjectively improved memory and 3 of the 7 who were subjectively unchanged were euthymic at follow-up.
Imaging Findings
White matter hyperintensities were observed in 11 of the 14 patients by a neuroradiologist unaware of their clinical features. These were multiple in 3 patients, but only 1 of these patients showed cognitive impairment when first tested.
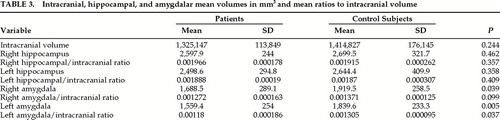
Intracranial and hippocampal volumes were not significantly different between patients and control subjects (Table 3). The volumes of the right and left amygdala were significantly smaller in the patient group, more so on the left, but when intracranial volume was corrected only the volume of the left amygdala remained significantly different. One patient had a much smaller left amygdala (1,030 mm3) compared with the mean for the group (1,560 mm3). The differences in left amygdalar volume between the patients and control subjects remained significant when this patient was excluded, but the difference between the left amygdala/ intracranial ratios was no longer significant (left amygdala, P=0.009; left amygdala/intracranial ratio, P=0.061).
The volumes of the hippocampus and the amygdala were not correlated with age in either group. There was a trend for the left and right hippocampus/intracranial ratios to be larger in patients euthymic at the time of the study (P=053 for both right and left). Other volumetric measurements were similar in the two groups.
There were no differences in intracranial or amygdalar volume between the 5 patients who had cognitive impairment initially and the rest of the group. Their left hippocampal/intracranial ratio was smaller compared with the rest of the group, but the difference fell short of statistical significance (initially impaired: mean=0.00176, SD=0.000125; others: mean=0.00196, SD=0.000190; P=0.062).
DISCUSSION
The salient finding of our study is the reduced amygdalar volume, especially on the left, in depressed patients with subjective memory difficulties. In addition, hippocampal changes may also be relevant in those who exhibit mild, albeit transient, objective cognitive impairment. The small sample size and the absence of a control group of depressed patients without subjective memory difficulties are the main shortcomings of the study.
The mild cognitive deficits present in some of our patients are in keeping with those described by others.1 All of our patients had subjective memory difficulties, but only a third showed mild objective intellectual or memory underfunctioning. This discrepancy between subjective and objective impairment is in agreement with previous reports8 of psychiatric morbidity as an independent predictor of memory impairment. Subjective memory complaints are considered to be inaccurate in predicting dementia,21 and our study supports these findings. Only half of our patients reported memory improvement, despite the normalization of objective performance. It can be argued that subjective “memory” complaints may reflect subtle abnormalities in memory or other cognitive skills not detected by our tests; alternatively, they could be explained as a combination of poor insight and depression-related negativism.
Some have reported cognitive improvement with the resolution of depression,22 but others3,4 have reported persistent memory and frontal/executive deficits in euthymic unipolar patients. Our study does not help to clarify this controversy, as cognitive improvement was observed even in those who remained depressed.
As a group our patients showed reduction in amygdalar volume, especially on the left. The reduction in amygdalar volume appears to be more closely related to depression than to cognitive impairment, although it is likely to be relevant to both. There is considerable evidence from animal and human studies23–25 that the amygdala plays a central role in the processing and modulation of emotion. It has been suggested6 that exposure to high levels of excitotoxic glucocorticoids may lead to amygdalar changes, which in turn may influence memory, attention, and perception through the connections with the hippocampus and orbitomedial cortex.
The small numbers involved in this study demand caution in interpreting volumetric differences between those with and without objective cognitive deficits, but it is of interest that hippocampal volume was smaller in the former. The role of the hippocampus in human memory is well known.26,27 Correlations between poor memory recall and loss of hippocampal volume has been described in a large population of euthymic, nondemented subjects,28 and our own studies29 have shown a correlation between decline in verbal and visual memory and loss of hippocampal volume in those at risk for Alzheimer's disease. On the other hand, studies of patients with affective illness have failed to show hippocampal changes,30 although loss of cortical gray matter in medial temporal structures may be associated with chronicity of depression.6
White matter hyperintensities were also present in our patients, but, in contrast with other reported findings,31 their severity was not related to the presence of objective cognitive changes or to poor outcome.
ACKNOWLEDGMENTS
Ms. Miriam Hall performed the neuropsychological testing. Prof. G. du Boulay reported on the MRI scans. Drs. W. Crum and L. Van der Tebartz provided tutorials on MRI volumetric measurements. Dr. J. Foong helped with statistical analysis. Prof. M. Rossor allowed us access to his patients. Prof. D. Miller and other members of the MS NMR Unit gave us access to the scanner.
Dr. von Gunten's work was supported by a grant from the Psychogeriatric Department of Lausanne University; Dr. Fox's by funding from the Medical Research Council; and Prof. Ron's by partial funding from the Scarfe Trust.
 |
 |
 |
1 Brown RG, Scott LC, Bench CJ, et al: Cognitive function in depression: its relationship to the presence and severity of intellectual decline. Psychol Med 1994; 24:829–847Crossref, Medline, Google Scholar
2 Burt DB, Zembar MJ, Niederehe G: Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull 1995; 117:285–305Crossref, Medline, Google Scholar
3 Marcos T, Salamero M, Gutiérrez F, et al: Cognitive dysfunctions in recovered melancholic patients. J Affect Disord 1994; 32:133–137Crossref, Medline, Google Scholar
4 Paradiso S, Lamberty GJ, Garvey MJ, et al: Cognitive impairment in the euthymic phase of chronic unipolar depression. J Nerv Ment Dis 1997; 185:748–754Crossref, Medline, Google Scholar
5 Sheline YI, Gado MH, Price JL: Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport 1998; 9:2023–2028Google Scholar
6 Sheline YI, Wang PW, Gado MH, et al: Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996; 93:3908–3913Google Scholar
7 Shah PJ, Ebmeier KP, Glabus M, et al: Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Br J Psychiatry 1998; 172:527–532Crossref, Medline, Google Scholar
8 Bassett SS, Folstein MF: Memory complaint, memory performance, and psychiatric diagnosis: a community study. J Geriatr Psychiatry Neurol 1993; 6:105–111Crossref, Medline, Google Scholar
9 O'Connor DW, Pollitt PA, Roth M, et al: Memory complaints and impairment in normal, depressed, and demented elderly persons identified in a community survey. Arch Gen Psychiatry 1990; 47:224–227Crossref, Medline, Google Scholar
10 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for clinicians. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
11 Zigmond AS, Snaith RP: The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67:361–370Crossref, Medline, Google Scholar
12 Wechsler DA: WAIS-R manual: The Wechsler Adult Intelligence Scale–Revised. New York, The Psychological Corporation, 1981Google Scholar
13 Warrington EK: Recognition Memory Test. Windsor, UK, NFER- Nelson, 1984Google Scholar
14 McKenna P, Warrington EK: The Graded Naming Test. Windsor, UK, NFER-Nelson, 1983Google Scholar
15 Nelson HE, Willison JR: The National Adult Reading Test, 2. Windsor, UK, NFER-Nelson, 1991Google Scholar
16 Warrington EK: The Camden Memory Tests. Hove, UK, Psychology Press, 1996Google Scholar
17 Raven JC: Standard Progressive Matrices. London, HK Lewis, 1938Google Scholar
18 Freeborough PA, Fox NC, Kitney RI: Interactive algorithms for the segmentation and quantification of 3-D MRI brain scans. Comput Methods Programs Biomed 1997; 53:15–25Crossref, Medline, Google Scholar
19 Duvernoy HM: The human hippocampus: an atlas of applied anatomy. Berlin, Springer-Verlag, 1988Google Scholar
20 Clegg F, Warrington EK: Four easy memory tests for older adults. Memory 1994; 2:167–182Crossref, Medline, Google Scholar
21 Tobiansky R, Blizard R, Livingston G, et al: The Gospel Oak Study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol Med 1995; 25:779–786Crossref, Medline, Google Scholar
22 Plotkin DA, Mintz J, Jarvik LF: Subjective memory complaints in geriatric depression. Am J Psychiatry 1985; 142:1103–1105Google Scholar
23 Adolphs R, Tranel D, Damasio H, et al: Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 1994; 372:669–672Crossref, Medline, Google Scholar
24 Le Doux J: The Emotional Brain. New York, Simon and Schuster, 1996Google Scholar
25 Morris JS, Öhman A, Dolan RJ: Conscious and unconscious emotional learning in the human amygdala. Nature 1998; 393:467–470Crossref, Medline, Google Scholar
26 Milner B: Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg 1972; 19:421–446Crossref, Medline, Google Scholar
27 Zola-Morgan S, Squire LR, Amaral DG: Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci 1986; 6:2950–2067Google Scholar
28 Golomb J, de Leon MJ, Kluger A, et al: Hippocampal atrophy in normal aging. Arch Neurol 1993; 50:967–973Crossref, Medline, Google Scholar
29 Fox NC, Warrington EK, Freeborough PA, et al: Presymptomatic hippocampal atrophy in Alzheimer's disease: a longitudinal MRI study. Brain 1996; 119:2001–2007Google Scholar
30 Hauser P, Altshuler LL, Berrettini W, et al: Temporal lobe measurement in primary affective disorder by magnetic resonance imaging. J Neuropsychiatry Clin Neurosci 1989; 1:128–134Link, Google Scholar
31 O'Brien J, Ames D, Chiu E, et al: Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: follow-up study. BMJ 1998; 317:982–984Crossref, Medline, Google Scholar



