An Update on Estrogen: Higher Cognitive Function, Receptor Mapping, Neurotrophic Effects
The sex steroids have been associated with brain development and global functioning directly or indirectly for thousands of years. Writings from the ancient Greeks and Egyptians linked emotional instability or any unacceptable behavior from a female with the uterus.1 Modern medicine continues to be perplexed by the influence of reproductive steroids on behavior. For example, a PubMed search of “estrogen and brain” gives 10,846 references since 1963. As 21st-century neuropsychiatrists try to understand the brain circuits and neurotransmitters that underlie emotion and behavior, the influence of sex steroids begins to establish its importance. Estrogen and mood is a much-studied area. Recent evidence indicates that at least a subset of depressive disorders are influenced by estrogen's effects at many points, particularly the premenstrual and perimenopausal times. A recent issue of a neuropsychiatric journal was dedicated to summarizing the current literature on the effects of estrogen on mood throughout the female life cycle.2 But what about estrogen's effects on other cortical functions? Cognition is an area of particular interest to the neuropsychiatrist. The current database and established truths for a relationship between cognition, neurodegeneration, and the sex steroids are much more limited. As our population ages and dementias become more prominent in our medical practices, it is vital that we more clearly understand any relationship between these steroids and neurodegeneration. The following discussion summarizes some of the most recent evidence linking one sex steroid, estrogen, with cognition and neurodegeneration.
ESTROGEN AND COGNITIVE FUNCTION
There have been numerous reports of an association between estrogen and performance on specific measures of higher cognitive function. In particular, higher estrogen levels have been associated with improvements in verbal performance and decrements in visual-spatial performance.3–5 However, a recent summary of the literature concluded that although there is observational evidence for estrogen enhancement of certain aspects of cognitive functioning, quantitative comparison across studies cannot be performed because of heterogeneity among subjects and variability in the cognitive measures used.6 A comparison of two recent studies illustrates this problem. Both were prospective studies in which performance on the Forward and Backward Digit Span test was compared in groups of postmenopausal females over time. In one, females receiving estrogen replacement therapy (ERT) performed better than females not receiving ERT at both study initiation and 18 months later.7 In the other, there were no differences in performance on this task, either at initiation or 2 years later.8 However, the second study matched participants for educational level. In general, females on ERT are better educated, have higher socioeconomic status, and are healthier than females who are not.9 Mood is also a critical variable that is sometimes overlooked. In most studies circulating levels of hormones have not been measured, making comparisons difficult. The situation is further complicated by the report that cerebrospinal fluid levels of estradiol (presumably representing hormone levels in the brain) are very different from circulating levels and do not decrease as much following menopause.10
ESTROGEN RECEPTOR MAPPING
In addition to the well-known localization of estrogen receptors within hypothalamic nuclei, which are important for regulation of sexual and reproductive behaviors (not illustrated), estrogen receptors have now been found in other areas of the brain (see Cover and Figure 1). There are at least two forms of the estrogen receptor (alpha and beta), and their distributions within the brain are different.11–13 Receptor mapping studies do not all agree, perhaps because several different techniques (autoradiography, in situ hybridization, immunocytochemistry) and multiple species (mouse, rat, guinea pig, monkey, human) have been used. There are clear species differences, so the following summary is based only on studies of human and nonhuman primates (see Cover and Figure 1). Several studies have found estrogen receptors in the hippocampal formation (hippocampus proper, dentate gyrus, subiculum, and entorhinal cortex), basal forebrain (septal nucleus, diagonal band of Broca, and nucleus basalis of Meynert), and mamillary bodies (Cover and Figure 1, blue areas).11,13–19 These support an influence on declarative (autobiographical, explicit) memory. Presence in the amygdala and dorsal raphe nucleus may underlie some of the effects of estrogen on mood and emotion (Cover and Figure 1, pink area).11,14–19 Estrogen receptors are present in movement-related areas including the substantia nigra and the subthalamic nucleus (Cover and Figure 1, green areas).13,17–19 They may also be present in the cerebellum (not illustrated), but exact localization is still unclear.11,13 Most studies have not found estrogen receptors in the basal ganglia (caudate, putamen, or globus pallidus).11,13–16,18,19 Several studies have reported estrogen receptors in various areas of the cerebral cortex (not illustrated), but this is still controversial.11,13,18 No obvious differences have been reported between females and males in regional distribution of the alpha estrogen receptor.15,16 There may be gender differences in the distribution of the beta form of the receptor.11 One study has reported similar distributions of estrogen receptors in intact and ovariectomized females.15
Estrogen has multiple modes of action within the central nervous system.12,20–24 It binds to a nuclear receptor, acting intracellularly to alter gene expression. Estrogen actions via this genomic mechanism, which are necessarily slow, may include inhibition of apoptosis, suppression of inflammatory reactions, and modulation of neurotrophins and growth factors as well as neuronal structure and synapse formation. Estrogen also has rapid actions, occurring far too quickly for genomic mechanisms. These may include its antioxidant effects as well as enhancement of cerebral blood flow and cerebral glucose utilization. These nongenomic effects probably occur via both plasma membrane receptors and non–receptor-mediated pathways. Some actions, such as modulation of neurotransmitters, may occur by both genomic and nongenomic mechanisms.
Estrogen interacts with multiple neurotransmitter systems at multiple sites. For instance, it has been shown to modulate the levels of dopamine (upregulation), serotonin (downregulation), norepinephrine (downregulation), and acetylcholine (upregulation) in prefrontal cortex.25 This may occur via direct actions within cortex, or indirectly via estrogen receptor–mediated changes in brainstem or basal forebrain areas. Estrogen co-localizes with some neurotransmitters. Estrogen receptors have been found in serotonergic neurons within the dorsal raphe nucleus, where estrogen appears to facilitate serotonergic transmission by several mechanisms.26 Estrogen alpha receptor–containing neurons in the amygdala are heavily invested with cholinergic terminals projecting from the basal forebrain (Figure 2).15 Estrogen modulates aspects of neuronal plasticity, including dendritic spine formation. In the hippocampus, for instance, estrogen receptors are localized in GABAergic interneurons. Estradiol exposure decreases the activity of these inhibitory interneurons (via an interaction with a neurotrophin), resulting in an increase in pyramidal cell excitability, which in turn promotes formation of new dendritic spines and synapses (Figure 3).27–29
NEUROTROPHIC AND NEUROPROTECTIVE EFFECTS OF ESTROGEN
Estrogen has neuroprotective and neurotrophic actions that may be mediated by a variety of routes. Estrogen receptors (both alpha and beta forms) have been found in microglia and within reactive astrocytes.30,31 In addition, there is evidence that estrogen suppresses activation of microglia and astrocytes and thereby the inflammatory cascade.31,32 Circulating estrogen is critical to the health of some types of neurons. This has been clearly demonstrated for a subpopulation of dopaminergic neurons in the substantia nigra, suggesting the importance of estrogen in Parkinson's disease.33 It is not yet clear whether this effect on dopaminergic neurons is mediated via the intracellular estrogen receptor or by a plasma membrane receptor for estradiol, although the antioxidant actions of estrogen have been implicated.21
Estrogen may also be important in other neurodegenerative diseases. Epidemiological studies generally support the view that estrogen replacement therapy (ERT) reduces the risk of developing Alzheimer's disease (AD),22–24,34,35 which occurs more frequently and progresses more quickly in females.23 It has been suggested that estrogen's anti-inflammatory action may be one important protective mechanism.31 The process is not yet clear, since there is not a decrease in cortical estradiol or testosterone in AD.36 The influence of ERT on the progression of neurodegeneration after onset is more controversial, as are its effects on cognition. There was an absence of therapeutic effect on measures of cognition, mood, and cerebral blood flow in a recent double-blind, placebo-controlled study of ERT in AD,37 suggesting that its therapeutic use needs further study.
The incidence of stroke is lower in premenopausal females than in other groups (males, postmenopausal females), perhaps a result of estrogen's influence on cerebral vasculature, its effect on circulating levels of cholesterol, and/or its antioxidant actions.20,23,38 Although some reports indicate ERT lowers the risk of stroke after menopause, not all studies agree.38 A recent large study actually found more clinically significant brain atrophy in postmenopausal females who were receiving ERT than in those who were not.39 In that study, the prevalence of infarcts as demonstrated by magnetic resonance imaging was not different between ERT and non-ERT groups, and measures of cognitive functioning did not correlate with duration of estrogen treatment. Similarly, although some studies have reported that females fare better than males following traumatic brain injury, a recent meta-analysis of the few studies available reporting outcome by gender found that females fared worse than males on virtually every outcome measure, with the interesting exception of “return to work.”40,41 Thus, although the neuroprotective actions of estrogen have been convincingly demonstrated in animal models of ischemia, contusion, hypoxia, and drug-induced toxicity, its protective effects in humans are less clear.20,23,24 There is a need for careful clinical studies in which potentially confounding factors such as premorbid conditions, severity of injury, and treatment differences can be assessed.
IMPLICATIONS
The influence of estrogen on such a wide range of brain functions has important implications for neuropsychiatry. It may be one reason why there are gender differences in both vulnerability to some mental illnesses and disease course. It suggests, as well, the potential for differences in response to therapeutics both across gender and as a function of life stage. The neuroprotective effects of estrogen raise the hope that it can be used to treat degenerative diseases of the CNS, such as Alzheimer's disease. It may also be helpful in salvaging tissue after stroke and traumatic brain injury. The existence of at least two forms of the estrogen receptor, and the differences in their distribution among brain areas, raises the possibility of designing pharmaceutical interventions that are targeted to specific aspects of estrogen's wide range of functions. It is clear, however, that estrogen has such widespread effects that it will be very difficult to predict what the effect of estrogen agonists and antagonists will be for particular aspects of cognitive function. Thus, new therapeutics will have to be very carefully evaluated.
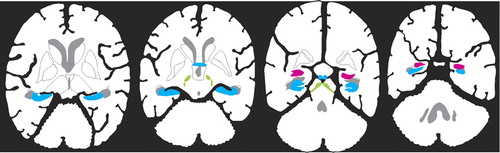
Figure 1. New areas in which estrogen receptors have been confirmed in primates are illustrated on a sagittal drawing of the human brain (Cover) and on axial human brain slices (Figure 1)Structures are color-coded by function: memory (blue: basal forebrain, hippocampal formation, mammillary body); emotion (pink: amygdala); movement (green: subthalamic nucleus, substantia nigra).
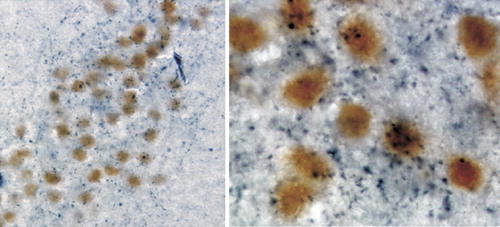
Figure 2. Immunohistochemical double labeling shows localization of the estrogen alpha receptor (yellow-brown label in neuronal nuclei) and choline aceytltransferase (blue label in cytoplasm) in the primate amygdalaNote that the co-association of cholinergic axon terminals with estrogen receptor–containing neuronal cell bodies is clear on the higher-magnification image (right).
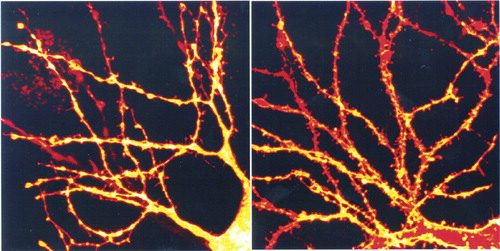
Figure 3. Photomicrographs of cultured hippocampal neurons immunohistochemically labeled with DiI (a plasma membrane dye) so that individual spine morphology could be visualized in the confocal microscopeThe neurons on the right have been exposed to estradiol (0.1 μg/ml) for 48 hours; the ones on the left have not. Note the marked increase of the number of mature spines on the dendrites of neurons exposed to estradiol.
1 Catonne JP: [Hippocratic concept of hysteria] (French). Ann Med Psychol (Paris) 1992; 150:705-719Medline, Google Scholar
2 Special Issue: Female-Specific Mood Disorders. CNS Spectrums: The International Journal of Neuropsychiatric Medicine 2001; 6:125-174Google Scholar
3 Sherwin BB: Estrogen effects on cognition in menopausal women. Neurology 1997; 48:S21-S26Google Scholar
4 Williams CL: Estrogen effects on cognition across the lifespan. Horm Behav 1998; 34:80-84Crossref, Medline, Google Scholar
5 Drake EB, Henderson VW, Stanczyk FZ, et al: Associations between circulating sex steroid hormones and cognition in normal elderly women. Neurology 2000; 54:599-603Crossref, Medline, Google Scholar
6 Haskell SG, Richardson ED, Horwitz RI: The effect of estrogen replacement therapy on cognitive function in women: a critical review of the literature. J Clin Epidemiol 1997; 50:1249-1264Google Scholar
7 Carlson LE, Sherwin BB: Higher levels of plasma estradiol and testosterone in healthy elderly men compared with age-matched women may protect aspects of explicit memory. Menopause 2000; 7:168-177Crossref, Medline, Google Scholar
8 Maki PM, Resnick SM: Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging 2000; 21:373-383Crossref, Medline, Google Scholar
9 Yaffe K, Grady D, Pressman A, et al: Serum estrogen levels, cognitive performance, and risk of cognitive decline in older community women. J Am Geriatr Soc 1998; 46:816-821Crossref, Medline, Google Scholar
10 Molnar G, Kassai-Bazsa Z: Gonadotropin, ACTH, prolactin, sexual steroid and cortisol levels in postmenopausal women's cerebrospinal fluid (CSF). Archives of Gerontology and Geriatrics 1997; 24:269-280Crossref, Medline, Google Scholar
11 Pau CY, Pau KY, Spies HG: Putative estrogen receptor beta and alpha mRNA expression in male and female rhesus macaques. Mol Cell Endocrinol 1998; 146:59-68Crossref, Medline, Google Scholar
12 McEwen BS: Clinical review 108: the molecular and neuroanatomical basis for estrogen effects in the central nervous system. J Clin Endocrinol Metab 1999; 84:1790-1797Google Scholar
13 Taylor AH, Al-Azzawi F: Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol 2000; 24:145-155Crossref, Medline, Google Scholar
14 Register TC, Shively CA, Lewis CE: Expression of estrogen receptor alpha and beta transcripts in female monkey hippocampus and hypothalamus. Brain Res 1998; 788:320-322Crossref, Medline, Google Scholar
15 Blurton-Jones MM, Roberts JA, Tuszynski MH: Estrogen receptor immunoreactivity in the adult primate brain: neuronal distribution and association with p75, trkA, and choline acetyltransferase. J Comp Neurol 1999; 405:529-542Crossref, Medline, Google Scholar
16 Donahue JE, Stopa EG, Chorsky RL, et al: Cells containing immunoreactive estrogen receptor-alpha in the human basal forebrain. Brain Res 2000; 856:142-151Crossref, Medline, Google Scholar
17 Gundlah C, Kohama SG, Mirkes SJ, et al: Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res 2000; 76:191-204Crossref, Medline, Google Scholar
18 Osterlund MK, Keller E, Hurd YL: The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience 2000; 95:333-342Crossref, Medline, Google Scholar
19 Osterlund MK, Grandien K, Keller E, et al: The human brain has distinct regional expression patterns of estrogen receptor alpha mRNA isoforms derived from alternative promoters. J Neurochem 2000; 75:1390-1397Google Scholar
20 Roof RL, Hall ED: Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma 2000; 17:367-388Crossref, Medline, Google Scholar
21 Sawada H, Shimohama S: Neuroprotective effects of estradiol in mesencephalic dopaminergic neurons. Neurosci Biobehav 2000; 24:143-147Crossref, Medline, Google Scholar
22 Birkhauser MH, Strnad J, Kampf C, et al: Oestrogens and Alzheimer's disease. Int J Geriatr Psychiatry 2000; 15:600-609Crossref, Medline, Google Scholar
23 Garcia-Segura LM, Azcoitia I, DonCarlos LL: Neuroprotection by estradiol. Prog Neurobiol 2001; 63:29-60Crossref, Medline, Google Scholar
24 Wise PM, Dubal DB, Wilson ME, et al: Estrogens: trophic and protective factors in the adult brain. Front Neuroendocrinol 2001; 22:33-66Crossref, Medline, Google Scholar
25 Kritzer MF, Kohama SG: Ovarian hormones differentially influence immunoreactivity for dopamine beta-hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol 1999; 409:438-451Crossref, Medline, Google Scholar
26 Bethea CL, Pecins-Thompson M, Schutzer WE, et al: Ovarian steroids and serotonin neural function. Mol Neurobiol 1998; 18:87-123Crossref, Medline, Google Scholar
27 Woolley CS, Weiland NG, McEwen BS, et al: Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci 1997; 17:1848-1859Google Scholar
28 Murphy DD, Cole NB, Greenberger V, et al: Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci 1998; 18:2550-2559Google Scholar
29 Murphy DD, Cole NB, Segal M: Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc Natl Acad Sci USA 1998; 95:11412-11417Google Scholar
30 Blurton-Jones M, Tuszynski MH: Reactive astrocytes express estrogen receptors in the injured primate brain. J Comp Neurol 2000; 433:115-123Crossref, Google Scholar
31 Vegeto E, Bonincontro C, Pollio G, et al: Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci 2001; 21:1809-1818Google Scholar
32 Garcia-Estrada J, Del Rio JA, Luquin S, et al: Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res 1993; 628:271-278Crossref, Medline, Google Scholar
33 Leranth C, Roth RH, Elswoth JD, et al: Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson's disease and memory. J Neurosci 2000; 20:8604-8609Google Scholar
34 Tang MX, Jacobs D, Stern Y, et al: Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 1996; 348:429-432Crossref, Medline, Google Scholar
35 Kawas C, Resnick S, Morrison A, et al: A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology 1997; 48:1517-1521Google Scholar
36 Twist SJ, Taylor GA, Weddell A, et al: Brain oestradiol and testosterone levels in Alzheimer's disease. Neurosci Lett 2000; 286:1-4Crossref, Medline, Google Scholar
37 Wang PN, Liao SQ, Liu RS, et al: Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology 2000; 54:2061-2066Google Scholar
38 Hurn PD, Macrae IM: Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab 2000; 20:631-652Crossref, Medline, Google Scholar
39 Luoto R, Manolio T, Meilahn E, et al: Estrogen replacement therapy and MRI-demonstrated cerebral infarcts, white matter changes, and brain atrophy in older women: the Cardiovascular Health Study. J Am Geriatr Soc 2000; 48:467-472Crossref, Medline, Google Scholar
40 Groswasser Z, Cohen M, Keren O: Female TBI patients recover better than males. Brain Inj 1998; 12:805-808Crossref, Medline, Google Scholar
41 Farace E, Alves WM: Do women fare worse: a meta-analysis of gender differences in traumatic brain injury outcome. J Neurosurg 2000; 93:539-545Crossref, Medline, Google Scholar



