Patterns of Change in the Treatment of Psychiatric Symptoms in Patients With Probable Alzheimer's Disease From 1983 to 2000
Abstract
The authors examined the pattern of use of psychiatric medication as prescribed by community physicians in 1,155 patients with probable Alzheimer's disease (AD) referred to the Alzheimer's Disease Research Center of Pittsburgh between April 1983 and July 2000. The use of antidepressants and of sedatives, hypnotics, and anxiolytics (SHA) increased over time, while the use of antipsychotics decreased. The increased use of antidepressants and decreased use of antipsychotics may reflect the growing evidence that newer antidepressants (e.g., selective serotonin reuptake inhibitors) can be used to treat not only mood-related disorders, but also abnormal behavior (e.g., aggression, agitation) and sleep disorders in AD. Although the use of SHA has a proven deleterious effect on patients with AD, their use has increased over the past two decades.
Psychiatric medications are used frequently to control different behavioral manifestations of Alzheimer's disease (AD). The effectiveness of these treatments has significant implications for the burden the disease puts on the patients, their caregivers, physicians, and society. Practice guidelines usually recommend using behavioral and environmental approaches first, followed by various medications.1 However, there is still considerable debate about the selection and effectiveness of various classes of medications to treat abnormal behavior (e.g., agitation, aggression, psychosis).2–7
Controlled trials conducted in the 1980s showed that no single antipsychotic or non-antipsychotic drug was best to treat abnormal behavior in patients with dementia, and drugs were only modestly superior to placebo.8,9 This controversy continued through the 1990s, with Teri et al.10 finding no significant differences among haloperidol, trazodone, behavioral management techniques, and placebo to treat agitation in AD. More recently, other studies did find that typical and atypical antipsychotics, as well as the new generation of antidepressants, are better than placebo for controlling agitation and aggression in AD patients.5,11–14
Over the past 20 years we have witnessed several important developments in the area of the treatment of the psychiatric manifestations of AD: 1) new antipsychotics13,15,16 that appear to have fewer side effects than traditional antipsychotics;17 2) a wide variety of new antidepressants that have proven efficacy in AD;18,19 and 3) a growing agreement among researchers that antidepressants can be used to treat not only depression, but also some abnormal behaviors.5,14,19
While the debate about the effectiveness of psychiatric medications, and the best ways to use them in the context of dementia, continues among researchers, little is known about how community physicians (including psychiatrists, neurologists, and primary care physicians) approach the treatment of psychiatric symptoms in AD. What is more important, little is known about how primary care physicians have incorporated recent scientific advances into their clinical practice. In this study, we examined how community physicians have changed their pattern of use of psychiatric medication over the past 20 years, with special emphasis on antidepressants, antipsychotics, and sedatives/hypnotics/anxiolytics (SHA).
METHODS
We reviewed the psychiatric medications used by 1,155 patients with the diagnosis of probable AD20 examined at the University of Pittsburgh Alzheimer's Disease Research Center (ADRC). Each participant in the study received an extensive neuropsychiatric evaluation, including medical history and physical examination, neurologic history and examination, semistructured psychiatric interview, and neuropsychological assessment. At the conclusion of these studies, each individual set of results was reviewed by the study team (neurologists, neuropsychologists, and psychiatrists) at a consensus conference. Details of our baseline and follow-up examination as well as our exclusion/inclusion criteria have been reported.21 Informed consent was obtained for all patients at study entry.
Psychiatric Examination
The psychiatric evaluations were conducted by geriatric psychiatrists using a semistructured interview22 that encompassed the DSM-III-R23 Axis I disorders. From 1991 on, all subjects were also assessed with the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Behavioral Rating Scale.24 In addition, the Hamilton Rating Scale for Depression (Ham-D)25 and the Blessed Dementia Rating Scale (BDRS) for activities of daily living26 interviews were completed by the psychiatrist on the basis of data from each patient and primary caregiver.
For the purpose of this study, we selected a group of psychiatric symptoms that usually require drug treatment. The criteria for the diagnosis of psychiatric symptoms have been reported previously.21 Briefly, the diagnosis of depression was made when patients met the DSM-IV criteria for major depression.27 Agitation occurred when patients had signs of emotional distress with increased motor activity. Aggression occurred when patients displayed verbal or physical aggressive behavior. Delusions were defined in accordance with the DSM-IV criteria27 and were distinguished from confabulations, disorientation, and amnesia by requiring that the false beliefs persist in spite of evidence of the contrary. Hallucinations were accepted as present if the patient had spontaneously reported a sensory perception with no concomitant external stimulus. Sleep disorders were considered to be present when patients had hypersomnia or insomnia.
Medication Use
Data on the psychiatric medications were gathered in two ways. First, the caregivers filled out a form that asked for the names (and doses) of past and present medications. Second, each patient brought in the actual pharmacy container of each drug, and this was reviewed by the neurologist and psychiatrist. These medications were prescribed by community family physicians or specialists who referred the patients to our clinic. Patients were referred to the ADRC as follows: 17% from university geriatric psychiatric clinics and 25% from university neurology clinics; 40% from various other sources (e.g., speaking engagements by the faculty, other health professionals); 1% from support groups; 5% referred because they have a family member with AD; and 12% self-referrals. The majority of the patients referred from nonacademic sources were followed by family practitioners. Because the most important advances in psychiatric treatment over the past 20 years have been made since 1990 (especially in the mid- and late 1990s), we have divided the observation period into three parts: 1983–1990, 1991–1995, and 1996–2000.
RESULTS
Of the 1,155 patients with a diagnosis of probable AD, 330 (28.5%) took psychiatric medications; 225 (19.5%) used antidepressants, 93 (8%) antipsychotics, and 76 (6.5%) SHA. Overall, there was an increase in the number of patients taking psychiatric medications from 1983 to 2000 (1983–1989, 23%; 1990–1995, 25%; 1996–2000, 37%; χ2=22.7, df=2, P<0.0001)
The demographic and neuropsychiatric characteristics of the patients per group are shown in Table 1 as a function of their date of study entry. Patients enrolled between 1983 and 1990 were younger and had a shorter duration of the symptoms of dementia, higher (i.e., worse) BDRS26 scores for activities of daily living, and lower Hachinski Ischemic Rating28 scores than those enrolled in 1991–1995 and 1996–2000. No statistical differences were noted among groups for scores on the Mini-Mental State Examination,29 Clinical Dementia Rating,30 Ham-D,25 or NYU Scale for Parkinsonism.31
Psychiatric Symptoms
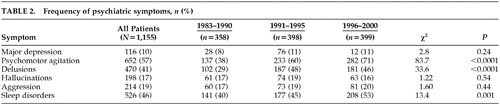
The frequency of major depression, aggression, and hallucinations was not statistically different between 1983 and 2000. By contrast, there were more patients with psychomotor agitation, delusions, and sleep disorders in 1991–2000 than in 1983–1990 (Table 2).
Use of Psychiatric Medication
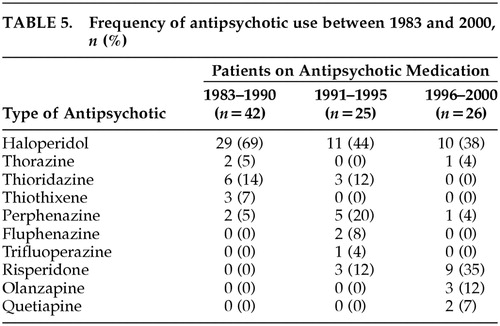
Table 3 shows the overall pattern of use of psychiatric medication over the past two decades. In 1983–1990, fewer patients were using antidepressants and more were using antipsychotics than in the 1991–1995 and 1996–2000 periods. In 1996–2000, more patients were taking SHA than in 1983–1990 and 1991–1995, and the proportion of patients taking selective serotonin reuptake inhibitors (SSRIs) and new antidepressants (NA; e.g., nefazodone) had increased since 1991. Notably, the proportion of patients taking atypical antipsychotics in the 1996–2000 period was similar to the proportion taking typical antipsychotics. The data on specific medications taken during the different time periods are shown in Tables 4, 5, and 6.
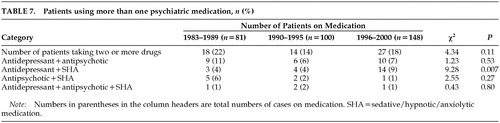
Table 7 shows the proportion of patients who were treated with more than one psychiatric drug. There were more patients using antidepressants with SHA in the 1996–2000 period than in the 1983–1990 and 1991–1995 periods.
DISCUSSION
This study showed that 28.5% of the studied patients with probable AD were taking some psychoactive medications at the time of entry into our registry. Of all the patients, 19.5% used antidepressants, 8% antipsychotics, and 6.5% SHA. However, the pattern of medications prescribed by community physicians was different in the 1990s compared with the 1980s, showing decreased use of antipsychotics and increased use of antidepressants and SHA. These findings are consistent with those of Mendez et al.,32 who found in about 1990 that 7% of the AD patients used antidepressants, 11.5% antipsychotics, and 7% SHA.
The increased use of antidepressants in AD may reflect evidence that these agents can treat syndromes other than depression, such as psychosis, agitation, and sleep disturbances.5,12,19 As noted here, while the frequency of major depression among the studied patients remained stable from 1983 to 2000, the frequency of psychosis, agitation, and sleep disturbances increased, and there was an associated increased in antidepressant use. Antidepressants can be used for longer periods of time and with less risk of the development of extrapyramidal signs than antypsychotics.17 Furthermore, although old (e.g., tricyclic) and new antidepressants seem to be equally efficacious in the treatment of depression, there are differences in their metabolism (cytochrome P450 inhibition), their potency-inhibiting neurotransmitter reuptake, and their potency for blocking neurotransmitter receptors. In particular, antimuscarinic effects are substantially lower with SSRIs and NA in comparison to tricyclics, with a decreased risk for cognitive toxicity in AD subjects. These differences seem to be well understood by community physicians, and 96% of the patients treated in 1996–2000 were on SSRIs or NA, although a small group was still on traditional antidepressants, perhaps reflecting a treatment failure of the newer antidepressants. This change in the pattern of SSRIs use has also been reported in population studies: Ganguli et al.33 found that 84.6% of the participants taking antidepressants were on tricyclics and 2.6% on SSRIs in 1987, whereas in 1996, 45.5% were on tricyclics and 36.4% on SSRIs.
Even though the new antipsychotic medications have fewer extrapyramidal side effects than typical antipsychotics, there has been an overall reduction in the use of any antipsychotics over the past 20 years. This may be explained, in part, by the increased use of antidepressants in the treatment of abnormal behavior in AD, at least in patients with mild/moderate stages. However, the decrease in antipsychotic use (from 12% to 7%) is more than offset by the increasing use of antidepressants (from 12% to 29%). A change in the pattern of drug use to treat behavioral problems does not fully account for this increase, suggesting that there might also be an increase in the use of antidepressants to treat mood disorders among patients with dementia.
Several studies have reported that new antidepressants (e.g., SSRIs)5,14 as well as typical11 and atypical12,13,15,16 antipsychotics are effective in the treatment of agitation and aggression in AD. However, whether antidepressants are better than antipsychotics in the treatment of psychiatric symptoms in AD is under debate. Sultzer et al.34 found no differences between the efficacy of haloperidol and trazodone in the treatment of agitation in AD patients.
Despite the recent advances in the development of new antipsychotics, 47% of the patients in the 1996–2000 period continued using typical antypsychotics. A possible explanation is that physicians are more comfortable treating patients with typical antipsychotics and that these medications have wider dosing ranges and more formulations than atypical antipsychotics (i.e., the availability of concentrates, injectables, tablets, sprinkling preparations, and the option of incorporating medication into food and drink).
SHA are the oldest and best established treatments for abnormal behavior and insomnia in demented and nondemented patients, and guidelines for the use of SHA in AD recommended short-term use of short-acting benzodiazepines.35 However, SHA have proven deleterious effects in elderly patients. Benzodiazepines can produce amnesia,36 increase the risk of falls and death,37,38 and increase costs of medical treatments.39 We have reported that the use of SHA is associated with reduced time to death in probable AD patients.40 Despite robust data that indicate SHA should be used judiciously in AD patients, their use has increased over the past 20 years in this cohort. Indeed, we found an increased SHA use during the observation period (from 4.5% in 1983–1990 to 9% in 1996–2000), consistent with recent observations from population studies showing an increase from 2.2% in 1987 to 10.7% in 1999.41
It is common to use more than one psychiatric medication, especially in patients with severe behavioral symptomatology, although there are no guidelines that recommend the best approach in these cases. In this case series, approximately 19% of the patients taking psychiatric drugs were using two or more drugs. Interestingly, there was an increased tendency to use antidepressants and SHA together, which may reflect the reduced sedative effects of SSRIs and NA relative to tricyclics.
This is an observational study on a referral cohort of AD patients, and thus our results may be biased toward a specific subgroup of patients. However, populations studies have reported similar findings in terms of increased antidepressant and SHA use over the past two decades.33,41 Another factor that may have affected our results is the change in the characteristics of the patients over these referral periods. As shown here, we examined patients with fewer disruptive behaviors in 1983–1990 than in 1991–2000, although they did not have significant differences in cognitive functions (MMSE, CDR scores). An increasing tendency to prescribe antidepressants for these behaviors could explain, in part, the better functional (BDRS) scores observed in the 1991–2000 period.
There have been significant advances in psychopharmacology over the past two decades. Since the 1980s, we have witnessed the introduction of a great number of successful antidepressants, and from the mid-1990s, the introduction of atypical antipsychotics. By contrast, the introduction of new sedatives/hypnotics/anxiolytics has not been as successful as other medications, although some SHA with shorter half-life have become available (e.g., alprazolam). These new medication opportunities are reflected in the way that physicians treat their patients, especially with the use of antidepressants. However, more controlled trials will be necessary to determine when an antidepressant is better than an antipsychotic for specific symptoms. There is a need for better dissemination of the advantages of atypical antipsychotics over conventional neuroleptics in elderly patients, and for the development of guidelines for combinations of neuropsychiatric medications. The expectations of families and patients have risen over the past 10 years as advances in AD treatment have been disseminated to the public. Direct-to-consumer advertising for many of these medications has created educated consumers who know what to “ask for.” These changes will also have an effect on the prescribing habits of physicians treating AD.
ACKNOWLEDGMENTS
This study was supported by Grants AG03705 and AG05133 from the National Institute on Aging. J.T.B. is the recipient of a Research Scientist Development Award, Level II (KO2-MH01077).
 |
 |
 |
 |
 |
 |
 |
1 Rabins PV: The treatment of noncognitive symptoms, in Alzheimer Disease, 2nd ed. Edited by Terry RD, Katzman R, Bick KL, et al. Philadelphia, Lippincott Williams and Wilkins, 2000, pp 415-421Google Scholar
2 Lemke MR: Effect of carbamazepine on agitation in Alzheimer's inpatients refractory to neuroleptics. J Clin Psychiatry 1995; 56:354-357Medline, Google Scholar
3 Gleason RP, Schneider LS: Carbamazepine treatment of agitation in Alzheimer's outpatients refractory to neuroleptics. J Clin Psychiatry 1990; 51:115-118Medline, Google Scholar
4 Porsteinsson AP, Tariot PN, Erb R, et al: An open trial of valproate for agitation in geriatric neuropsychiatric disorders. Am J Geriatr Psychiatry 1997; 5:344-351Crossref, Medline, Google Scholar
5 Nyth AL, Gottfries CG: The clinical efficacy of citalopram in treatment of emotional disturbances in dementia disorders: a Nordic multicenter study. Br J Psychiatry 1990; 157:894-901Crossref, Medline, Google Scholar
6 Stern RG, Dyffelmeyer ME, Zemishlani Z, et al: The use of benzodiazepines in the management of behavioral symptoms in demented patients. Psychiatr Clin North Am 1991; 14:375-384Crossref, Medline, Google Scholar
7 Weiler PG, Mungas D, Bernick C: Propranolol for the control of disruptive behavior in senile dementia. J Geriatr Psychiatry Neurol 1988; 1:226-230Crossref, Medline, Google Scholar
8 Schneider LS, Sobin PB: Non-neuroleptic medications in the management of agitation in Alzheimer's disease and other dementia: a selective review. Int J Geriatr Psychiatry 1991; 6:691-708Crossref, Google Scholar
9 Schneider LS, Pollock VE, Lyness SA: A metaanalysis of controlled trials of neuroleptic treatment of dementia. J Am Geriatr Soc 1990; 38:553-563Crossref, Medline, Google Scholar
10 Teri L, Logsdon RG, Peskind E, et al: Treatment of agitation in AD: a randomized, placebo-controlled clinical trial. Neurology 2000; 55:1271-1278Crossref, Medline, Google Scholar
11 Devenand DP, Sackeim HA, Brown RP: A pilot study of haloperidol treatment of psychosis and behavioral disturbance in Alzheimer's disease. Arch Neurol 1989; 46:854-857Crossref, Medline, Google Scholar
12 DeDeyn PP, Rabheru K, Rasmussen A, et al: A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology 1999; 53:946-955Crossref, Medline, Google Scholar
13 Street JS, Clark WS, Gannon KS, et al: Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch Gen Psychiatry 2000; 57:968-976Crossref, Medline, Google Scholar
14 Pollock BG, Mulsant BH, Sweet RA, et al: An open pilot study of citalopram for behavioral disturbances of dementia: plasma levels and real-time observations. Am J Geriatr Psychiatry 1997; 5:70-78Crossref, Medline, Google Scholar
15 Parsa MA, Greenaway HE, Bastani B: Quetiapine for the treatment of psychosis in Lewy body disease. Neurology 2000; 54(suppl 3):A451-A452Google Scholar
16 Katz IR, Jeste DV, Mintzer JE, et al: Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry 1999; 60:107-115Crossref, Medline, Google Scholar
17 Jeste DV, Okamoto A, Napolitano J, et al: Low incidence of persistent tardive dyskinesia in elderly patients with dementia treated with risperidone. Am J Psychiatry 2000; 157:1150-1155Crossref, Medline, Google Scholar
18 Lyketsos CG, Sheppard J-ME, Steele CD, et al: Randomized, placebo-controlled, double-blind clinical trial of sertraline in the treatment of depression complicating Alzheimer disease: initial results from the Depression in Alzheimer's Disease Study. Am J Psychiatry 2000; 157:1686-1689Crossref, Medline, Google Scholar
19 Houlihan DJ, Mulsant BH, Sweet RA, et al: A naturalistic study of trazodone in the treatment of behavioral complications of dementia. Am J Geriatr Psychiatry 1994; 2:78-85Crossref, Medline, Google Scholar
20 McKhann G, Drachman DA, Folstein MF, et al: Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984; 34:939-944Crossref, Medline, Google Scholar
21 Lopez OL, Becker JT, Klunk W, et al: Research evaluation and diagnosis of probable Alzheimer's disease over the last two decades, I. Neurology 2000; 55:1854-1862Crossref, Medline, Google Scholar
22 Mezzich JE, Dow JT, Cottman GA: Developing an information system for a comprehensive psychiatric institute, I: principles, design, and organization. Behavior Research Methods and Instrumentation 1981; 13:459-463Crossref, Google Scholar
23 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 3rd ed., revised. Washington, DC, American Psychiatric Press, 1987Google Scholar
24 Tariot PN, Mack JL, Patterson MB, et al: The behavior rating scale for dementia of the Consortium to Establish a Registry for Alzheimer's Disease. Am J Psychiatry 1995; 152:1349-1357Crossref, Medline, Google Scholar
25 Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
26 Blessed G, Tomlinson BE, Roth M: The association between quantitative measures of dementia and senile changes in the cerebral white matter of elderly subjects. Br J Psychiatry 1968; 114:797-811Crossref, Medline, Google Scholar
27 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Press, 1994Google Scholar
28 Hachinski VC, Iliff LD, Zihka E, et al: Cerebral blood flow in dementia. Arch Neurol 1975; 32:632-637Crossref, Medline, Google Scholar
29 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
30 Hughes CP, Berg L, Danzinger WL: A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140:566-572Crossref, Medline, Google Scholar
31 Hoehn M, Yahr M: Parkinsonism: onset, progression, and mortality. Neurology 1967; 17:427-442Crossref, Medline, Google Scholar
32 Mendez MF, Martin RJ, Smyth KA, et al: Psychiatric symptoms associated with Alzheimer's disease. J Neuropsychiatry Clin Neurosci 1990; 2:28-33Link, Google Scholar
33 Ganguli M, Mulsant B, Richards S, et al: Antidepressant use over time in a rural older adult population: the MoVIES Project. J Am Geriatr Soc 1997; 45:1501-1503Crossref, Medline, Google Scholar
34 Sultzer DL, Gray KF, Gunay I, et al: A double-blind comparison of trazodone and haloperidol for treatment of agitation in patients with dementia. Am J Geriatr Psychiatry 1997; 5:60-69Crossref, Medline, Google Scholar
35 Borson S, Raskind MA: Clinical features and pharmacologic treatment of behavioral symptoms of Alzheimer's disease. Neurology 1997; 48(5, suppl 6):S17-S24Google Scholar
36 Curran HV, Bond A, O'Sullivan G, et al: Memory functions, alprazolam and exposure therapy: a controlled longitudinal study of agoraphobia with panic disorder. Psychol Med 1994; 24:969-976Crossref, Medline, Google Scholar
37 Robin DW, Hasan SS, Edeki T, et al: Increased baseline sway contributes to increased losses of balance in older people following triazolam. J Am Geriatr Soc 1996; 44:300-304Crossref, Medline, Google Scholar
38 Wysowski DK, Baum C, Ferguson WJ, et al: Sedative-hypnotic drugs and the risk of hip fracture. J Clin Epidemiol 1996; 49:111-113Crossref, Medline, Google Scholar
39 Yuen EJ, Zisselman MH, Louis DZ, et al: Sedative-hypnotic use by the elderly: effects on hospital length of stay and costs. Journal of Mental Health Administration 1997; 24:90-97Medline, Google Scholar
40 Lopez OL, Wisniewski SR, Becker JT, et al: Psychiatric medication and abnormal behavior as predictors of progression in probable Alzheimer's disease. Arch Neurol 1999; 56:1266-1272Crossref, Medline, Google Scholar
41 Basu R, Dodge H, Ganguli M: Sedative-hypnotic use over ten years in a rural older adult community sample. Presented at the 14th Annual Meeting of the Association of Geriatric Psychiatry, San Francisco, CA, February 24, 2001Google Scholar



