A CNV-Distraction Paradigm in Combat Veterans With Posttraumatic Stress Disorder
Abstract
Fourteen veterans with posttraumatic stress disorder (PTSD) and 14 without PTSD participated in a contingent negative variation (CNV)-distraction paradigm. Subjects were instructed to press a button after hearing a high-pitched tone (S2) preceded by a low-pitched tone (S1). One-half of the trials included a white-noise distracter placed in the S1–S2 interval. Posttraumatic stress disorder subjects had larger frontal, but smaller central and parietal CNVs, regardless of condition (distracter, no distracter) or epoch (early CNV, late CNV). In PTSD subjects, the N1/P2 complex was smaller to warning (S1) and distracter stimuli and did not show the extent of facilitation present in non-PTSD subjects. Findings highlight PTSD-related differences in phasic cortical excitability and attention.
Posttraumatic stress disorder (PTSD) is a common psychiatric response to a severely stressful experience.1 Symptoms associated with the disorder include disturbances in attentional processes, and it is believed that these disturbances play a role in the psychological, occupational, and social impairment present in the disorder.
Given that PTSD clearly has both psychological and biological components, tools such as event-related potentials (ERPs) that can investigate both components are important in understanding the disorder. Event-related potentials are segments of scalp-recorded electroencephalograms (EEG) that are time-locked to actual or anticipated stimuli and can provide an ongoing measure of electrical neural activity. In this study, two ERP components, the N1/P2 complex, and the contingent negative variation (CNV), were studied in order to index aspects of cortical excitability and attention in combat veterans.
Studies of the CNV are highly relevant in understanding PTSD and common symptoms of the disorder such as attention-related problems, anhedonia, and hyperarousal. This is because the CNV represents underlying neurophysiology that may correlate with these common problems. Neurophysiologically, it is believed that the CNV reflects broad cortical excitability, the activation of which is presumed necessary for optimal processing of anticipated and incoming stimuli. Reduction in such activation would imply difficulties maintaining motivation and adequate cognitive resources to sustain concentration for demanding or complex attentional tasks.
The CNV was first reported by Walter2 as a voltage negativity that appears when an individual is regularly presented with an imperative stimulus (S2) subsequent to a warning stimulus (S1), such that the occurrence of an S2 is contingent upon the presentation of an S1. In the S1–S2 interval, the CNV is seen as a growing negativity starting approximately 400 msec after the presentation of the warning stimulus and resolving at the imperative stimulus. While there has been some debate regarding the functional correlates of CNV amplitude, the negativity associated with the CNV is often thought to represent increased cortical excitability in preparation of a motor act or decision making.3
One theory of CNV amplitude, proposed by Tecce and Cole,4 has received considerable interest. Tecce and Cole's “attention-distraction” hypothesis suggests that CNV amplitude is related to attention in a linear fashion, such that amplitude of the CNV increases as attention to the task increases. This conclusion is drawn from a number of studies using a CNV-distraction paradigm. These studies have shown that extraneous or distracting stimuli presented in the S1–S2 interval (or just prior to S1) suppress CNV development.5,6,7,8 Tecce and Scheff6 propose that task irrelevant stimuli reduce CNV development because they interfere with attention via distraction. A review of distraction studies by Tecce and Cattanach9 has shown that there are few subsequent studies challenging this hypothesis.
A distraction paradigm, such as the one discussed above, would have considerable value in studying attention in individuals with PTSD. Posttraumatic stress disorder is characterized by difficulties attending to task relevant stimuli (i.e., concentration difficulties) as well as a tendency to over-attend to threatening and trauma-relevant stimuli (i.e., hypervigilance). Group differences between non-PTSD and PTSD participants in a distraction-CNV paradigm could highlight both baseline attentional problems, as manifested by smaller CNVs during nondistracter trials, on top of tendencies toward distractibility, as manifested by further CNV reductions during distracter trials.
In addition, a distraction paradigm allows for the unique opportunity to investigate possible facilatory effects of the CNV on stimuli presented during the CNV interval. In particular, the peak-to-peak amplitudes measured from the N1 to the P2 (the N1/P2 complex) has been shown to be reliably larger to stimuli (sometimes referred to as “probes”) that occur during the S1–S2 interval, as compared to stimuli that occur between trials.3,10,11,12 Rockstroh et al.10 concluded that the cortical excitability associated with CNV activity enhances the processing of stimuli presented during the CNV. In a CNV-distraction paradigm such as the one used in this study, an N1/P2 complex is elicited by both the S1 stimulus as well as the distracting stimulus presented in the S1–S2 interval. Facilitation of stimulus processing would be reflected in a larger N1/P2 complex to the distracting stimulus, given that the distracting stimulus occurs while the CNV is present. In clinical populations, such as individuals with PTSD, a lack of such facilitation during the CNV interval would suggest an inability to maintain an optimal level of cortical excitability to process stimuli efficiently.
To date, there has been only one CNV study published that examines PTSD-diagnosed participants.13 In this study, CNV amplitudes were significantly smaller in subjects with PTSD compared to control subjects, both high and low in traumatic life events. The authors speculated that this reduction in CNV in PTSD patients might have been related to a lack of motivation to perform the experimental task and the disruption in frontal noradrenergic processes.
In the current study, combat veterans with and without PTSD pressed a button to the imperative (S2) stimulus during an S1–S2 task. In order to assess possible PTSD-related differences in distractibility and facilitation, one-half the trials included a task-irrelevant sound in the S1–S2 interval. We predicted smaller overall CNV amplitudes in subjects with PTSD. Evidence of PTSD-related distraction would be supported by the smallest CNV amplitudes being present in PTSD subjects during the CNV interval occurring after the presentation of the distracting stimulus. We also predicted that smaller CNV amplitudes in PTSD subjects would result in smaller facilitory effects on the N1/P2 complex.
METHOD
Participants
Twenty-eight Vietnam veterans were enrolled in the study, 14 of whom had a diagnosis of PTSD and 14 had not been diagnosed with PTSD. Subjects were recruited from newspaper advertisements and fliers. A preliminary phone screen excluded those with diagnoses of substance abuse or dependence within the past year, histories of epilepsy or severe head trauma, hearing impairments, and neurological or medical disorders that might interfere with neurological functioning.
Procedure
After giving informed consent, subjects underwent PTSD diagnostic evaluation. Posttraumatic stress disorder diagnosis was determined using the Clinician Administered PTSD Scale (CAPS)14 for DSM-IV. The Combat Exposure Scale (CES)15 was used to assess exposure to combat.
On the second day of testing, veterans returned to participate in the electroencephalographic (EEG) portion of the study. Wearing an elastic EEG cap (Electro-cap International: Eaton, OH), each participant performed the CNV task. With electrical grounding to the head and a common reference to the left mastoid, EEG was recorded from 18 scalp locations (F7, F3, FZ, F4, F8, T7, C3, CZ, C4, T8, P7, P3, PZ, P4, P8, O1, OZ, O2). Vertical electro-oculogram (vEOG) was measured from a tin electrode placed below the left eye and compared to EEG electrodes placed above the left eye (i.e., FP1), and the horizontal electro-oculogram (hEOG) was measured from an electrode placed on the right canthus and EEG electrodes on the left side of the head (i.e, F7). All electrode impedances were less than 5 kOhms. The continuous recording of electrical activity on the scalp was band-pass filtered at 0.01-100 Hz, amplified 50,000 times by an SA Instrumentation D.C. Amplifier (San Diego, CA), and sampled at 256 Hz. Trials with blinks or substantial eye movement artifact were not included in the individual averages. Analyses revealed that the two groups did not differ with respect to the number of trials lost to artifact (M = 2.84, SD = 1.85). Data were digitally low pass filtered at 12 Hz/12dB, high pass filtered at 0.01 Hz/12 dB, and averaged according to stimulus with InstEP Systems software (Ottawa, Canada).
Participants were instructed to focus on a white sticker that was 2 centimeters in diameter and placed on a screen 1.5 meters away. Etymotic Research ER-3 Tubephone earphones were placed in each ear. Sixty trials, each composed of a 50-msec 1,000 Hz “warning” tone (S1) that was followed 1,500 msec later with a 50-msec 2,000 Hz “imperative” tone (S2) were presented. Each tone had a rise/fall time of 5 msec, and the dB level was set at 85. Participants were asked to press a button following the S2 stimulus. The distracter sound was identical to the S1 stimulus in rise/fall time and amplitude and differed with respect to the S1 only in its representation of multiple frequencies (i.e., white noise). The distracter was presented 750 msec after the onset of the warning tone (S1). With an intertrial interval of 6, 8, or 10 seconds, thirty distracter trials were randomly interspersed with 30 nondistracter trials.
Scoring
Individual subjects averaged CNV amplitudes and reaction times were computed for the distracter and nondistracter conditions from blink-free trials. Amplitude scoring produced average voltages, relative to a 250 msec baseline prior to S1, at each electrode between 500 and 750 msec post-S1 for the early CNV component and 1,250 and 1,500 msec post-S1 for the late CNV component. These intervals were chosen in order to score the CNV while avoiding overlap with the distracting stimulus. Means and standard deviations were calculated for the following montages: frontal (F7, F3, FZ, F4, F8), central (T7, C3, CZ, C4, T8), and parietal (P7, P3, PZ, P4, P8). N1/P2 peak to peak amplitudes for the warning stimulus were measured from the most negative peak between 75 and 125 msec to the subsequent most positive peak between 150 and 300 msec. N1/P2 peak to peak amplitudes for the distracting stimulus were measured from the most negative peak 75 to 125 msec after the distracter and the subsequent most positive peak between 150 and 300 msec following the distracter.
Planned Analyses
Contingent negative variation amplitudes were analyzed using a 2 × 2 × 2 × 3 mixed model analysis of variance (ANOVA) with group (PTSD, non-PTSD) as the between subjects factor and condition (distracter, nondistracter), epoch (early and late) and region (frontal, central, parietal) as the within subjects factors. During the distracting trials, N1/P2 amplitudes were analyzed using a 2 × 2 × 3 mixed model ANOVA with group (PTSD, non-PTSD) as the between subjects factor and stimulus (S1, distracter) and region (frontal, central, parietal) as the within subjects factors. All reported degrees of freedom and subsequent p values are based on adjustments made using the Greenhouse-Geisser correction in the repeated-measures design. An alpha level of 0.05 was used for all statistical tests. Given previous reductions in CNV amplitude found in the literature and our specific unidirectional hypotheses, 1-tailed tests of significance are reported.
RESULTS
Subject Demographics and Behavioral Responses
Age (M = 52.4, SD = 4.8) and educational level (M = 8.0, SD = 2.2, “partial college”) did not differ between the groups. As expected, PTSD veterans displayed higher scores on Cluster B (re-experiencing) [t (26) = 13.27, p < 0.001], Cluster C (avoidance), [t (26) = 9.94, p < 0.001], and Cluster D (hyperarousal), [t (26) = 11.46, p < .001] of the CAPS. Among the 14 PTSD subjects, 11 were on psychotropic medication; eight were prescribed selective serotonin reuptake inhibitors; five were prescribed benzodiazepines; four were prescribed tricyclic antidepressants; one was prescribed an atypical antidepressant; and one was taking a prescription antihistimine. Although all subjects were exposed to at least “light” combat according to the CES, PTSD veterans reported significantly higher exposure to combat than did the non-PTSD group [t (26) = 2.83, p < 0.01]. There were no group differences in reaction time (M = 347.0, SD = 140.6), failures to respond (M = 0.4, SD = 0.9), early responses (M = 1.3, SD = 1.3), or number of trials lost to artifact (M = 2.8, SD = 1.8).
CNV Amplitudes
The four-way ANOVA revealed a significant group × region effect (F = 6.5, df = 1,26, p = 0.01) with relatively larger (more negative) CNV amplitudes at frontal sites in the PTSD subjects and smaller (less negative) CNVs at central and parietal sites, regardless of epoch (early or late) or condition (distracter or nondistracter). [See Figure 1 and Figure 2]. Group did not interact with condition and epoch, [group × condition × epoch, F (1,26) = 0.036, p > 0.05], and thus our hypothesis that postdistracter CNV amplitudes would be differentially affected in PTSD subjects by the stimulus placed in the S1–S2 interval was not reinforced.
To further investigate the significant group × region effect, three follow-up four-way (group × condition × epoch × region) ANOVAs were performed, but with only two levels of the region variable. One ANOVA compared frontal and central regions, one compared frontal and parietal regions, and another compared central and parietal regions. The group × region interaction was significant in the ANOVA that compared frontal regions and central regions [Group × region, F (1,25) = 6.4, p = 0.009] and parietal and frontal regions [group × region, F (1,26) = 3.37, p = 0.04]. CNV or contingent negative variation difference scores (central-frontal amplitudes) were calculated to further investigate frontal relative to central CNV amplitudes.4,16,17,18 There was a significant main effect for group with considerably smaller CNV difference scores in PTSD subjects for the early CNV, [F (1,26) = 5.12, p = 0.02], the late distracter CNV, [F (1,26) = 5.32, p = 0.02] and the late no distracter CNV, [F = (1,26)4.80, p = 0.01].
N1/P2 Amplitudes
The planned three-way ANOVA (group × stimulus × region) revealed a main effect for stimulus with larger N1/P2 peak to peak amplitudes to the distracting stimulus, as compared to the S1 stimulus [F (1,26) = 14.7, p = 0.005], supporting the notion that the N1/P2 complex was facilitated during the CNV interval (see Figure 2). A main effect for group [F (1,26) = 10.3, p = 0.002] was modified by a group × stimulus interaction with the non-PTSD subjects showing a larger increase in N1/P2 amplitudes from the S1 stimulus to the distracting stimulus [F (1,26) = 3.0, p = 0.05]. For region, there was no main effect on N1/P2 amplitudes, nor did it interact with any other factor.
DISCUSSION
The findings in this study indicate that subjects with chronic PTSD show electrophysiological evidence consistent with differences in cortical excitability that may result in inadequate processing of environmental stimuli. Even in this cued reaction time task, in which the subjects are forewarned about the need to respond to an anticipated stimulus, patterns of activation differed in PTSD subjects, and responses to the study stimuli were attenuated. While biases to trauma-relevant information in attentional allocation in PTSD are well established, this study adds to growing evidence suggesting that there are deficits in stimulus processing at the most fundamental level. Reductions in cortical excitability in combination with N1/P2 reductions to both S1 and S2 stimuli likely reflect an inability to maintain motivation and concentration for a relatively neutral, sustained, and repetitive task.
While CNV amplitudes were reduced across the scalp in PTSD subjects, these subjects did show larger frontal CNV amplitudes relative to central and parietal amplitudes. Although the exact significance of this pattern in CNV has yet to be clearly delineated in PTSD, similar CNV difference scores have been found in psychotic patients,16,17 schizophrenics,4,12 victims of head trauma,18 and obsessive-compulsive patients.19 The fact that such a pattern is present in a number of severe psychiatric disorders suggests that PTSD may have commonalities with these disorders not previously appreciated. Prior investigators generally have interpreted such increased frontal CNVs as representative of increased frontal activation due to inefficient frontal functioning20 and found that such ratios correlate with performance on a test of frontal functioning. While the heightened frontal amplitudes do not directly indicate an underlying frontal hyperactivation, it is worthy to note that findings are consistent with a growing literature that establishes the presence of frontal lobe abnormalities in subjects with PTSD.21
Our data did not demonstrate that the distracter stimulus differentially affected CNV amplitudes in subjects with PTSD. We did find that both groups demonstrated the typical experimental effect, with smaller CNV after the distracter, as compared to before the distracter, but the PTSD subjects did not show significantly larger reductions. It is possible that PTSD-specific effects were not present in PTSD subjects, as the white noise distracter was not particularly potent or particularly relevant to PTSD-specific pathology. Future CNV-distraction paradigms in PTSD may benefit from varying the intensity, the novelty, and the trauma content of the distracting stimulus.
Recent evidence indicates that stimuli processed during CNV intervals are processed more intensely—suggesting the increased excitability of the cortical networks and specific gain control properties of the CNV.3,12 In this study, both the PTSD group and the non-PTSD group demonstrated evidence of facilitation with larger N1/P2 complexes to the distracter relative to the S1 warning stimulus. However, we found significantly stronger facilitation effects among non-PTSD subjects. This finding, in addition to N1/P2 amplitude reductions to the distracting stimulus indicates that this stimulus was not fully processed at a basic sensory level by PTSD subjects. It is possible that the relatively smaller CNV amplitudes in PTSD subjects may result in suboptimal levels of cortical excitability to fully process the stimulus occurring in the CNV interval.
There were limitations to the study that should be considered in the interpretation of the data. Most studies that assess facilitation effects compare identical stimuli presented during the CNV interval and between the S1–S2 trials. Our S1 stimulus was a pure tone, while our distracter was a white noise segment. While the white noise was of equal intensity, duration, and rise/fall time as the S1 stimulus, it is possible that the smaller facilitation effect in PTSD subjects may have been due to a lack of responsiveness to the differing physical properties of the white noise stimulus (i.e., presence of all frequencies versus one frequency) or the different context (warning versus distracter) assigned to each stimulus. Additionally, our subjects were not examined for illicit or undisclosed substance use through urine analysis or blood testing. The study relied on subject self-reports, and thus it can not be stated decisively that the result may have been influenced by current drug use. Further, eleven of our 14 PTSD subjects were taking prescribed psychotropic medications although it is likely that the medications being taken would have worked against the reported effects (i.e., normalize PTSD ERPs).
Future Directions
The fact that individuals with PTSD are hypothesized to have increases in attention to threatening stimuli is an area for future investigation. Of particular interest is that some studies have shown patients with specific phobias to generate larger CNVs in anticipation of phobogenic S2 presentation22 and are larger to fear-relevant stimuli during conditioning tasks.23 Investigating whether PTSD patients would generate larger CNV amplitudes in anticipation of trauma-relevant or threatening stimuli is a logical next step. In addition, utilizing trauma-relevant distracters that occur both between and during the CNV intervals might capture disorder-specific differences in distractibility. Finally, the evidence of differential CNV amplitude patterns in PTSD subjects that are consistent with patterns seen in other disorders thought to have inefficient frontal lobe functioning suggests that investigating neurological correlates of putative frontal tasks would be important in increasing our understanding of the cognitive and physiological features of the disorder.
ACKNOWLEDGMENTS
This project was funded by a Veterans Administration Merit Review Entry Grant to Matthew Kimble and National Institute of Mental Health FIRST Award R29 MH58215.
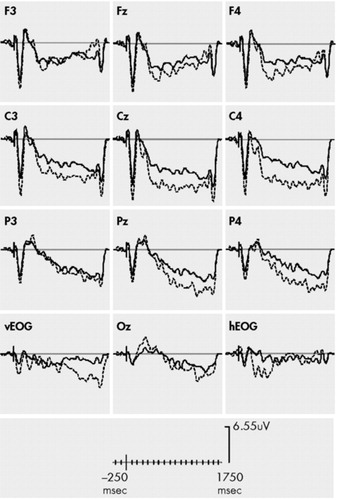
FIGURE 1. CNV Amplitudes During No Distracter Trials in PTSD (solid) and No PTSD (dashed) Subjects
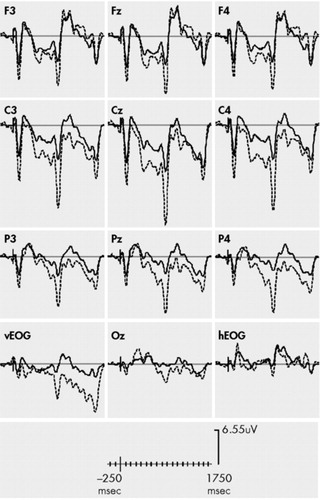
FIGURE 2. CNV Amplitudes During Distracter Trials in PTSD (solid) and No PTSD (dashed) Subjects
1 The Diagnostic and Statistical Manual of Mental Disorders, 4th edition, edited by the American Psychiatric Association. Washington, DC, American Psychiatric Association 1994Google Scholar
2 Walter WG: The contingent negative variation. An electrical sign of significance of association in the human brain. Science 1964; 146:434Google Scholar
3 Rockstroh B, Mueller M, Wagner M, et al: Event related and motor responses to probes in a forewarned reaction time task in schizophrenic patients. Schizophr Res 1994; 13:23–34Crossref, Medline, Google Scholar
4 Tecce JJ, Cole JO: Psychophysiologic responses of schizophrenics to drugs. Psychopharmacologia 1972; 24:159–200Crossref, Medline, Google Scholar
5 McCallum WC, Walter WG: The effects of attention and distraction on the CNV in normal and neurotic subjects. Electroencephalogr Clin Neurophysiol 1968; 25:319–329Crossref, Medline, Google Scholar
6 Tecce JJ, Scheff NM: Attention reduction and suppressed direct-current potentials in the human brain. Science 1969; 164:331–333Crossref, Medline, Google Scholar
7 Otto DA, Benigus VA, Ryan LJ, et al: Slow potential components of stimulus, response, and preparatory processes: a multiple linear regression model. In Progress in Clinical Neurophysiology, Vol. 1, Attention, voluntary contraction and event-related potentials, edited by Desmedt JE. Basel, Switzerland, Karger, 1977, pp 211–230Google Scholar
8 Tecce JJ, Hamilton BT: CNV reduction by sustained cognitive activity (distraction). In Event related slow potentials in the brain: Their relation to behavior, Suppl 33, edited by McCallum WC, Knotts JR. Amsterdam, Elsevier, 1973, pp 229–237Google Scholar
9 Tecce JJ, Cattanach L: Contingent Negative Variation. In Electroencephalography: Basic Principles, Clinical Applications and Related Fields. Edited by Niedermeyer E, Lopes da Silva F. Baltimore, Urban and Schwarzenberg, 1993, pp 887–910Google Scholar
10 Rockstroh B, Muller M, Wagner M, et al: “Probing” the nature of the CNV. Electroencephalogr Clin Neurophysiol 1993; 87:235–241Crossref, Medline, Google Scholar
11 Wagner M, Alho D, Lavikainen J, et al: Sequential analysis of pitch discrimination and ERPs during auditory selective attention and distraction: evidence for facilitation and inhibition. In New Developments in Event-Related Potentials. Edited by Heinze HJ, Munte TF, Mangun GR. Boston, Birkhauser, 1993, pp 203–213Google Scholar
12 Wagner M, Rendtorff N, Kathman N, et al: CNV, PINV, and probe-evoked potentials in schizophrenics. Electroencephalogr Clin Neurophysiol 1996; 98:130–143Crossref, Medline, Google Scholar
13 Boudarene M, Timsit-Berthier M: Interest of events-related potential in assessment of posttraumatic stress disorder. In Psychobiology of Posttraumatic Stress. Edited by Yehuda R, McFarlane A. New York, Annals of The New York Academy of Sciences, 1997, pp 492–497Google Scholar
14 Blake D, Weathers FW, Nagy LM, et al: The development of Clinician-Administered PTSD Scale. J Trauma Stress 1995; 8:75–90Crossref, Medline, Google Scholar
15 Keane TM, Fairbank JA, Caddell JM, et al: Clinical evaluation of a measure to assess combat exposure. Psychological Assessment: J Consult Clin Psychol 1989; 1:53–55Crossref, Google Scholar
16 van den Bosch RJ: Contingent negative variation and psychopathology: frontal-central distribution. Biol Psychiatry 1983; 18:615–634Medline, Google Scholar
17 van den Bosch RJ: Contingent negative variation: components and scalp distribution in psychiatric patients. Biol Psychiatry 1984; 19:963–972Medline, Google Scholar
18 Tecce JJ, Cattanach L: Contingent negative variation. In Electroencephalography: Basic Principles, Clinical Applications and Related Fields, edited by Niedermeyer E, Lopes da Silva F. Baltimore, Urban and Schwarzenberg, 1987, pp 657–679Google Scholar
19 Sartory G, Master D: Contigent negative variation in obsessive-compulsive patients. Biol Psychol 1984; 18:253–267Crossref, Medline, Google Scholar
20 van den Bosch RJ, Rozendaal N, Mol JM: Slow potential correlated of frontal function, psychosis, and negative symptoms. Psychiatry Res 1988; 23:201–208Crossref, Medline, Google Scholar
21 Charney DS, Deutch AY, Southwick SM, et al: Neural circuits and mechanisms of post-traumatic stress disorder. In Neurobiological and Clinical Consequences of Stress: From Normal to Post-Traumatic Stress Disorder. Edited by Friedman M, Charney DS, Deutch A. Philadelphia, Lippincott-Raven, 1995 pp 271–287Google Scholar
22 Lumsden J, Howard RC, Fenton GW: The contingent negative variation (CNV) to fear-related stimuli in acquisition and extinction. Int J Psychophysiol 1986; 3:253–261Crossref, Medline, Google Scholar
23 Regan M, Howard R: Fear conditioning, preparedness, and the contingent negative variation. Psychophysiology 1995; 32:309–214Crossref, Google Scholar



