Risk-Taking on Tests Sensitive to Ventromedial Prefrontal Cortex Dysfunction Predicts Early Relapse in Alcohol Dependency: A Pilot Study
Abstract
Twenty one patients in a residential rehabilitation program fulfilling International Classification of Diseases-10 (ICD) criteria for alcohol dependence syndrome were recruited. On neuropsychological tests, alcohol dependent patients relapsed early if they made choices governed by immediate gain irrespective of later outcome, which is consistent with dysfunctional ventromedial-prefrontal cortex mediating the inability to resist the impulse to drink despite ultimately deleterious effects. The authors suggest that the use of neuropsychological tasks of decision making may have several advantages over more conventional strategies for studying alcoholism.
The prefrontal cortex is particularly vulnerable to damage following long-term alcohol abuse.1 Studies have found executive dysfunction, which is believed to be associated with prefrontal cortex impairment, in abstinent alcohol-dependent subjects in association with reduced frontal metabolism.2 The consequences of executive dysfunction include the failure to plan ahead or to think adequately about the consequences of actions. In alcohol dependence, such deficits may cause early relapse because individuals are unable to develop and use strategies to avoid alcohol or because they are unable to withhold the immediate desire to drink for long-term benefits. We tested these hypotheses using tests of executive function in recently detoxified alcohol-dependent subjects and, during a 3-month period, compared those who relapsed with those who remained abstinent. We used a test of planning sensitive to dorsolateral-prefrontal cortex function3 and tests of decision making or gambling believed to reflect the integrity of ventromedial-prefrontal cortex.4,5 We also measured memory and personality traits, which can be abnormal in alcoholism and may contribute to early relapse.
METHOD
Subjects
Twenty-one patients in a residential rehabilitation program fulfilling International Classification of Diseases-10 (ICD) criteria for alcohol dependence syndrome participated. Exclusion criteria included polysubstance dependency, organic brain disease, learning difficulties, and comorbid mental illness. Wandsworth Local Research Ethics Committee approved the study, and subjects gave written informed consent. Assessments were performed after 21 days of detoxification. Twenty normal volunteers served as comparison subjects for the neuropsychological tests and personality assessments.
Assessments
Three computerized executive tests were administered. 1) A computerized version of the Tower of London Planning Task,6 which requires subjects to plan and execute a series of moves in order to change the position of balls hanging in billiard pockets to match a goal arrangement, was administered. The number of required moves on each trial varies between 3 and 5. The measure is the number of solutions (out of 12) solved in the minimum number of moves (perfect solutions). 2) A decision making task7 in which subjects decided whether a token was hidden in a blue or red box was also administered. Subjects were given “odds” information (9:1, 8:2, 7:3, 6:4) to guide them. On each of 72 trials, they could also gain or lose points by betting according to their certainty of making a correct choice. The measures were the percent trials in which they chose the correct box (quality) and the percent accumulated points bet at each odd (risk). 3) A gambling task4 in which subjects selected a card from one of four decks on each of 100 trials was administered. Each card turn was rewarded with points, but some cards subsequently demanded a penalty forfeit of points. The decks were arranged so that the cards in two of the decks resulted in higher points than cards in other decks, but they extracted higher penalties and were disadvantageous compared to the “good” decks in the long term. Memory was assessed using a computerized paired associates test.8 Subjects needed to learn the position of six or eight patterns presented on the screen one at a time. They were reminded of their mistakes and had up to 10 attempts to correctly recall the position of each pattern. Measures were total number of errors (learning) and number of pattern location recalled correctly at the first attempt (memory). All subjects completed the computerized tasks except the decision making task, which was completed by all comparison subjects and 16 patients. Intelligence quotient (IQ) was estimated using the National Adult Reading Test,9 which was completed by 18 comparison and 16 patients. Other measures were: the Structured Clinical Interview for DSMIII-R, antisocial and borderline subscales; the Brief Psychiatric Rating Scale (BPRS); the Addiction Severity Index (ASI), which was completed by all patients; the Barratt Impulsiveness Scale (BIS) (34-item),10 (see Table) which was completed by 18 patients and 19 comparison subjects; and the Dimensional Assessment of Personality–Basic Questionnaire (DAPP-BQ),11 completed by 20 patients and all comparison subjects.
Because of small numbers, group differences were analyzed using the Mann-Whitney U Test, except the decision making task, which was analyzed using analysis of variance (ANOVA) due to repeated measures. All probabilities are two-tailed.
RESULTS
Although the patients had a higher mean IQ (mean (SD), comparison group: 112.44 (8.49), patients: 119.44 (10.19); U= 62.5, p=0.005) and were slightly older (comparison group: 36.05 (10.97), patients: 40.95 (9.47), N=140.00, p=0.068), there were no differences in paired associates (memory: N=208, p=0.97; learning: U=194, p=0.68) and planning (perfect solutions: U=165, p=0.24). There were trends for the alcoholics to risk more points than controls across all odds on the decision making task (N=2.74, df=1,34, p=0.11) and to select more cards from the “bad” decks on the gambling task (N=142, p=0.08). Patients were more impulsive on the BIS (U=55, p<0.001) and had higher scores on emotional dysregulation (U=76.5, p=0.001), dissocial behavior (U=84.5, p=0.002) and compulsive (U=108.5, p=0.01) DAPP-BQ personality factors.
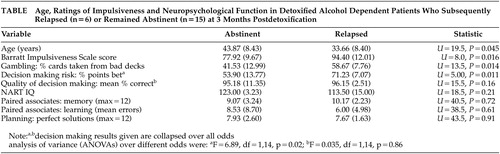
The patients were divided into those who had relapsed (N=6) or remained abstinent (N=15) at 3 months. The table shows that the relapsed patients were significantly younger, were more impulsive on the BIS, sampled significantly more cards from the bad decks on the gambling task and consistently risked more points across all odds on the decision making task. There were no differences between the groups on IQ, planning, quality of decision making, learning and memory. Six patients reached DSMIII-R criteria for borderline or dissocial personality disorder, with equal numbers in each group (Chi-Square =0.6, df=1, nsec). There were no differences between the groups with respect to DAPP-BQ personality factors (range of U values: 24–34), years drinking to intoxication (N=35, p=0.43) and psychopathology (BPRS, N=32.5, p=0.33).
DISCUSSION
Recently detoxified alcohol dependent patients were more likely to relapse within 3 months if they made more choices in which the immediate reward was large but the long term consequences were disadvantageous on a gambling task, and if they staked more points on their decisions being correct on a decision making task. It is unlikely that these findings reflect alcohol-induced brain damage because they were unimpaired on a memory test sensitive to the early stages of dementia.8 Long-term alcoholism particularly affects dorsolateral-prefrontal cortex1 and our patients were also unimpaired on a test of planning, sensitive to damage of this area.3,6 This specific pattern of performance is reminiscent of patients with ventromedial-prefrontal cortex lesions, who perform normally on most neuropsychological tests yet are impaired on decision making tasks where performance requires the weighing-up of risks and benefits.4 On such tasks they behave as if they are insensitive to the future consequences of their decisions and make choices based on immediate effects.4 Thus, relapsed patients displayed a pattern of behavior governed by the reinforcing effects of immediate rewards which in everyday situations may prevent them from resisting the impulse to drink, despite the ultimately deleterious effects. This substantiates findings made by Bechara et al. on a mixed group of alcohol and stimulant abusers12 in that our relapsed patients may represent a subgroup of alcohol dependent individuals with a specific premorbid dysfunction of ventromedial-prefrontal cortex.
Emotionally unstable and antisocial behavior has long been recognized as a risk factor for subsequent alcoholism. Compared to abstinent patients, relapsed patients were younger and scored higher on ratings of impulsiveness—features of Cloninger Type II alcoholism.13 However, although our patients had a high frequency of personality disorder (29%) and displayed higher than normal ratings of personality dimensions considered to reflect emotional instability/harm avoidance and dissocial behavior/sensation seeking,11,13 these measures did not distinguish those who relapsed or abstained. This is consistent with other studies, which have failed to characterize alcoholic patients using personality assessments.14
Although these results are from a pilot study and need to be replicated in a much larger group of patients, we tentatively suggest that the use of neuropsychological tasks of decision making may have two advantages over more conventional strategies for investigating alcoholism. First, these tasks may be more sensitive to behaviors fundamental to subtypes of alcoholism. Second, these tasks provide a neuroscience framework for the investigation of the neurobiological substrates of addiction, for example, abnormalities of serotonin function have been linked with impulsive behavior and alcoholism.15 In alcoholics, investigation of the serotoninergic modulation of ventromedial-prefrontal cortex function, in association with performance on these tasks, may provide an important avenue for further research.7
ACKNOWLEDGMENTS
Dr. Bowden-Jones was supported by a Priory Hospital Fellowship.
Findings in this study were presented at the 26th Annual Research Society on Alcoholism Scientific Meeting, Ft Lauderdale, FL, June 21–25, 2003.
 |
1 Kril J, Halliday G, Svoboda M, Cartwright H: The cerebral cortex is damaged in chronic alcoholics. Neuroscience 1997; 79:983–998Crossref, Medline, Google Scholar
2 Dao-Castellana M, Samson Y, Legault F, Martinot J, Aubin H, Crouzel C, Feldman L, Barrucand D, Rancurel G, Feline A, Syrota A: Frontal dysfunction in neurology normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med 1998; 28:1039–1048Crossref, Medline, Google Scholar
3 Owen AM, Doyon J, Petrides M, Evans AC: Planning and spatial working memory examined with a positron emission tomography study in humans. Eur J Neuroscience 1996; 8:353–364Crossref, Medline, Google Scholar
4 Bechara A, Damasio AR, Damasio H, Anderson SW: Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994; 50:7–15Crossref, Medline, Google Scholar
5 Rogers R, Owen A, Middleton H, Williams E, Pickard J, Sahakian B, Robbins T: Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital cortex. J Neuroscience 1999; 19:9029–9038Crossref, Medline, Google Scholar
6 Owen A, Downes J, Sahakian B, Polkey C, Robbins T: Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 1990; 28:1021–1034Crossref, Medline, Google Scholar
7 Rogers R, Everitt B, Baldacchino A, Johnson A, Swainson R, London M, Deakin J, Sahakian B, Robbins T: Dissociable effects in the decision-making of chronic amphetamine abusers, opiate users, patients with focal damage to prefrontal cortex, tryptophan normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 2000; 20:322–329Crossref, Google Scholar
8 Fowler K, Saling M, Conway E, Semple J, Louis W: Paired associate performance inthe early detection of DAT. J the Int Neuropsychol Soc 2002; 8:58–71Crossref, Medline, Google Scholar
9 Nelson H, Willison J: The revised National Adult Reading Test (NART)-Test Manual. Windsor, Berks, NFER-NELSON, 1991Google Scholar
10 Patton J, Stanford M, ES B: Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol 1995; 51:768–774Crossref, Medline, Google Scholar
11 Livesley WJ, Lang KL, Vernon PA: Phenotypic and genetic structure of traits delineating personality disorder. Arch Gen Psychiatry 1998; 55:941–948Crossref, Medline, Google Scholar
12 Bechara A, Dolan S, Denburg N, Hindes A, Anderson S, Nathan P: Decision-making deficits linked to a dysfunctional ventromedial prefrontalcortex, revealed in alcohol and stimulant abusers. Neuropsychologia 2001; 39:376–389Crossref, Medline, Google Scholar
13 Cloninger CR: Neurogenetic adaptive mechanisms in alcoholism. Science 1987; 236:410–416Crossref, Medline, Google Scholar
14 Mulder R: Alcoholism and personality. Aust NZ J Psychiatry 2002; 36:44–52Crossref, Medline, Google Scholar
15 Higley D, Linnoila M: A non-human primate model of excessive alcohol intake. personality and neurobiological parallels of type I and type II-like alcoholism. Rec Dev Alcohol 1997; 13:191–219Medline, Google Scholar



