Feeling Down: Idiom or Nature?
Emotional experiences, in contrast to communication (expression and perception of emotional faces and prosody), are differentiated along a lateral neuroanatomical axis, such that the left hemisphere mediates emotions with positive valence together with approach behaviors and the right mediates emotions with negative valence together with withdrawal behaviors. 1 , 2 For example, deactivation of the left hemisphere through injection of barbiturates often generates crying and sadness; deactivation of the right hemisphere produces laughter and an elevated mood. 3 Left frontal lobe strokes induce depression (the catastrophic reaction), and strokes of the right frontal lobes induce euphoria or indifference. 4 Further, functional imaging studies have also found significant activation at the left temporal and dorsolateral frontal regions in response to positive emotions and activation of the right temporal and dorsolateral frontal regions in response to negative emotions. 5
The left and right hemispheres also appear to have differential roles in the allocation of spatial actions-intentions and sensory attention (AIA), the left hemisphere allocating AIA to the right body centered hemispace and the right hemisphere primarily mediating attention and intention to left hemispace, but also to the right hemispace. 6 , 7 Based on the postulates that the left and right hemispheres mediate different emotional states and that each hemisphere directs AIA to contralateral space, we proposed that the experience of an emotion might alter the right-left balance of AIA.
In regard to the down-proximal (down) versus up-distal allocation of attention, the right hemisphere is also dominant for mediating AIA in distal-superior space and the left hemisphere in proximal-inferior space. 8 Thus, the influence of emotion on attentional allocation along a radial (transverse) or vertical (coronal) plane can also be determined by the balance of activation between the left and right hemispheres. Specifically, whereas positive emotions, which are associated with left hemisphere activation, should direct attention down-proximal, negative emotions associated with right hemisphere activation should direct attention up-distal.
There is, however, an alternative possibility. Mishkin and Ungerleider 9 coined the “what” and “where” systems as terms to describe the dorsal and ventral visual processing streams. Lesion studies of humans have revealed that parietal lesions often induce egocentric neglect and temporal lesions induce allocentric or object centered neglect. 10 In addition, it appears on the vertical and radial line bisections; with dorsal lesions there is neglect for lower space inducing a vertical superior and radial distal spatial bias, 11 and with more ventral lesions there is a bias to vertical-lower and radial proximal space. 12 In regard to emotions, with sadness and depression people are more likely to allocate their attention to their self, and with surprise, joy, and happiness they are more likely to allocate their attention to objects in their environment. Thus, it is possible that with happiness the ventral allocentric visual attentional system is activated and people attend upward and outward. In contrast, with sadness the dorsal stream is activated and people attend downward and inward.
We conducted an initial investigation examining whether positive and negative emotional expressions could generate such changes in the allocation of AIA. 13 Specifically, we instructed participants to place emotionally labeled wooden pegs on a felt covered board. The subjects were told that they could place these pegs in any board location of their choosing. The emotional labels included happy, surprise, joy, sad, afraid, and disgust. We found that all the pegs with positive emotional labels were placed in distal left hemispace and that the placement of all the negative emotional pegs was proximal and to the right of the positive pegs.
Although our initial investigation supported the idea that emotions can influence the allocation of AIA, there were several limitations of this prior study. We did not include a neutral control condition as a comparison. Hence, we could not ascertain whether the induced bias in AIA differed from a neutral condition or even a resting baseline condition. Additionally, we were concerned that the procedure for inducing emotion was potentially weak given that the procedure involved the use of emotionally labeled pegs. As a result of using this simple procedure, the intensity of the emotions aroused may have been lower than with other procedures commonly used to induce emotion. This initial investigation also did not employ a manipulation check to determine whether the participants were actually experiencing the desired emotion. Finally, our initial investigation used a pseudorandom order of presentation of emotions in that the participants were free to choose the order of placement of the emotional pegs.
In this study we sought to learn if we could support or provide converging evidence of this initial study when correcting for these limitations. Numerous prior studies have used emotional memories to successfully induce emotional experiences and induce changes in brain activity, 14 – 17 and thus, in this study we decided to use this emotional memory technique. In this current study, we also randomized the order of presentation and had the participants provide verbal descriptions of the emotional memories as well as indicate the age and intensity of the memories.
METHODS
Participants
A total of 20 individuals (12 women and eight men), with an age range of 23 to 43 years old (mean=29.55 years, SD=5.30), volunteered to participate. All participants were medical students or residents at two separate large university medical centers. The participants were all right handed as determined by self verbal report and had no history of depression, psychiatric illnesses, or neurological diseases, as assessed by verbal report.
Apparatus and Procedures
All participants were initially provided with a brief description of the experimental protocol and then signed Institutional Review Board informed consent agreeing to participate in this investigation. The participants were told that they would be required to recall a series of emotional memories, focusing on details, such as what happened, the people involved and how this experience make them feel. The emotional memories that the subjects were requested to recall included: surprise (something that had been unexpected), happy (something that made you happy or pleased you), funny (something that made you laugh), disgust (something that repulsed you), anger (something that made you mad), sad (something that made you sad and tearful), and a neutral memory (something that does not have an emotional component). Although we did not specify the content of the surprise memory, surprises can be either positive or negative, but all the subjects reported that their surprise memories were positive in valence. The neutral condition required the participants to recollect an event that did not arouse any emotion, as for example driving or eating breakfast. The potential confound from sequence effects was controlled by using a randomized order of presentation of the different memory conditions.
The participants were allowed to recall each emotional memory for one minute. After each emotional memory was recalled, the subjects were then provided with a 354 mm × 354 mm sheet of paper. The subjects were seated at a table, and the paper was presented in front of them on the tabletop. The participants’ head was not restrained, and they were able to look in any direction. The paper was situated so that the subjects’ midsagittal plane bisected this paper. Immediately after recalling the emotional memory, the subjects were asked to mark this paper by placing an “x” on the paper, and this “x” could be placed in any position they wished to place it.
After recollecting each emotional memory and placing their mark on the paper, we asked the subjects to provide a brief description of each emotional memory to be certain that the subjects understood the task and as a manipulation check to ensure that the subjects had, in fact, recalled an emotional memory of the desired valence. After each memory condition the participants were also asked to rate the intensity of the memory on a scale of 1 to 6 and to indicate the age of the memory. The age of the memory was converted to days for the purpose of conducting statistical analyses. After the subjects made their “x,” the paper was removed from the table and a new piece of paper was placed on the table for the next trial.
RESULTS
Each sheet of paper was first divided into quadrants by bisecting the paper both horizontally and vertically with straight lines. The distance from the horizontal midline (parallel but anterior to the subjects’ coronal plane) to each of the seven memory marks was calculated in millimeters, with negative values representing marks placed within the proximal or lower half and positive values representing marks placed within the distal or upper half of the sheet of paper. The horizontal distance from the vertical line (aligned with subjects’ midsagittal plane) to each of the memory marks was also measured, with negative values representing marks placed on the left half of the paper and positive values representing marks on the right half. These radial-vertical and horizontal right and left distances in millimeters were used as dependent variables for statistical analyses.
Data Analyses
The results of a 2 (position: radial-vertical and horizontal) × 7 (emotion: surprise, happy, funny, disgust, anger, sad, and neutral) repeated measures analysis of variance (ANOVA), with repeated factors of position and emotion, indicated a significant position × emotion interaction (F=3.264, df=6, 114, p=0.005). Whereas the main effect for emotion was not statistically significant, the main effect for position reached significance (F=4.762, df=1, 19, p=0.042). Multiple comparisons were then conducted to determine whether the positive and the negative emotions differed in their vertical-radial and horizontal (right-left) placements. Hence, the vertical-radial and horizontal placement of each positive emotion was compared to each negative emotion, using a Bonferroni correction for experiment wise error rate (p<0.0028 considered significant). Regarding differences in vertical-radial placement, the results indicated that the sad condition (mean=−29.23, SD=92.08) differed significantly from the positive-surprise condition (mean=48.83, SD=58.45; t=3.799, df=19, p=0.001; Cohen’s d=−1.01), the funny condition (mean=55.75, SD=74.72; t=3.887, df=19, p=0.001; Cohen’s d=−1.01), and the happy condition (mean=56.28, SD=53.26; t=4.114, df=19, p=0.001; Cohen’s d=−1.14). Additionally, the anger condition (mean=−19.50, SD=69.20) differed significantly from the happy condition (mean=56.28, SD=53.26; t=4.495, df=19, p=10.0003; Cohen’s d=−1.23). None of the other vertical-radial comparisons and none of the horizontal comparisons were significant. See Figure 2 for placement of each emotion.
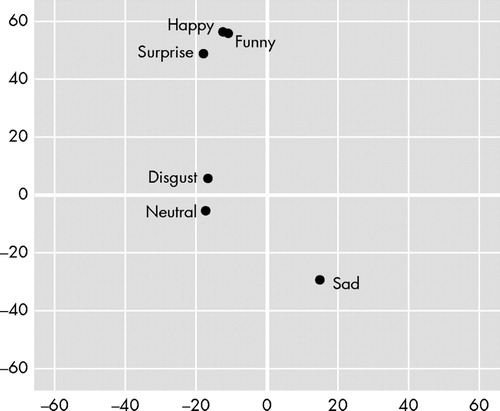
We also wanted to determine whether the placement of each emotion differed from zero (i.e., the position where the vertical-radial and horizontal bisection line intersected). Hence, we conducted a series of one-sample t -tests using zero as the test value for the vertical-radial and horizontal placements for each emotion. Bonferroni’s correction was used to control for experiment wise error rate (p<0.0036 considered significant). The results indicated that the surprise (t=3.736, df=19, p=0.001), funny (t=3.337, df=19, p=0.003), and happy (t=4.725, df=19, p=0.0002) conditions all differed from the horizontal midline. No other vertical comparisons and none of the horizontal comparisons were significant.
Finally, given the potential confounds of emotional memory intensity and age, we conducted all possible comparisons between the seven emotion conditions to examine if any of the conditions differed in terms of intensity or age. As before, Bonferroni’s correction was used to control for experiment wise error rate (p<0.0024 considered significant). The results indicated no significant differences among any of the emotion conditions in terms of either intensity or age.
DISCUSSION
Our results indicate that when subjects placed marks during all three positive emotional memories (happy, funny, and surprise), their marks were placed more distally or higher than the marks they made during negative-sad emotional memories. In addition, the happy memories were also placed more distally than the angry memories. During the recall of all three positive emotional memories, the subjects placed their marks distal to an imaginary line that went through the vertical-radial midline of the response page. Due to the small sample size these results are considered preliminary. They are, however, similar to our previous investigation, 13 in which we also found that all the pegs indicating positive emotions were placed distally to all the pegs indicating negative emotions and that the positive emotions were placed significantly distal (superior) to the vertical radial midline of the board. Hence, experiencing positive versus negative emotions induces different spatial biases in the transverse body plane along the proximal (low)-distal (high) axis.
In contrast to our prior study, 13 our results did not demonstrate that experience of either positive or negative emotions induced a systematic right or left spatial bias. One possible reason for the lack of significant differences in the right-left horizontal axis might be related to statistical power. Our previous study did find right-left asymmetries, and perhaps in this current study if our sample size was increased to match that of our previous investigation, we would have found significant differences in the right-left axis. Additionally, whereas in this current study the size of the paper on which the marks were placed measured 354 mm × 354 mm (13.875” × 13.875”), the board in our first was nearly twice as large, measuring 24” × 24” (609.60 mm × 609.6 mm). The smaller response space in this current study might have confined our subjects’ lateral response biases.
The previous study also used different methods than this current study, and this procedural alteration might have accounted for the difference between studies. In the prior study the subjects were asked to place emotionally labeled pegs, and in this current study the subjects were more likely to experience emotions. Recollection of emotional memories may result in a different pattern of cerebral activity. Generally, retrieval of autobiographical memories is associated with prefrontal cortex activation. 18 Daselaar et al. 19 also reported hippocampal and prefrontal involvement in the recollection of autobiographical memories, and the retrieval of memories for emotional events is associated with activation of the amygdala, hippocampus, and prefrontal cortex. 20 Further research will need to be conducted to determine whether these different methods of inducing emotions generate different influences on the spatial allocation of AIA. Support for this possibility is provided by research indicating that different methods of inducing emotions generate different patterns of psychophysiological activity 21 and different patterns of neurophysiological activity. 17
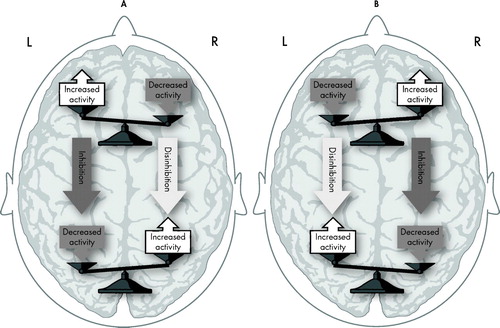
In this prior study 13 a leftward AIA bias was found to be associated with positive emotions and the rightward AIA bias with negative emotions. This direction of bias is opposite that which would be predicted by the left-positive versus right-negative hemispheric emotion model. In an attempt to explain these right-negative, left-positive associations Foster et al. 13 suggested an interhemispheric and intrahemispheric inhibition model. For example, positive emotions would activate the left frontal lobe, and this activation would induce inhibition of the right frontal lobe and left parietal lobe. Inhibition of the right frontal lobe and left parietal lobes would disinhibit the right parietal lobe, and an activated right parietal lobe would direct AIA to left space ( Figure 1 ). However, since we did not find any right or left asymmetries in this study, our results do not provide support for this prior model.
The reason for the significant radial-vertical differences between the negative and positive emotions might be related to the use of an arbitrary spatial notation system used to express the emotional experiences (i.e., positive up and negative [sad] down). An alternative explanation might be that the recollection of emotional memories and the experience of emotions may have altered the patterns of brain activation, and these alterations of brain activation might have induced spatial attentional and/or action-intentional biases. As mentioned, lesion studies of humans have revealed that parietal lesions often induce egocentric neglect and temporal lesions induce allocentric or object centered neglect. 10 In addition, it appears that on the vertical and radial line bisections, with dorsal lesions there is neglect for lower space inducing a vertical superior and radial distal spatial bias, 11 and with more ventral lesions there is a bias to vertical-lower and radial proximal space. 12 Mishkin and Ungerleider 9 coined the “what” and “where” systems as terms to describe the dorsal and ventral visual processing streams. In regard to emotions, with sadness and depression people are more likely to allocate their attention to their self, and with surprise, joy and happiness they are more likely to allocate their attention to objects in their environment. Thus, it is possible that with happiness the ventral allocentric visual attentional system is activated and people attend upward and outward. In contrast, with sadness the dorsal stream is activated and people attend downward and inward. This possible explanation of our results will, however, have to be further tested.
Although the results of this study increase our knowledge about how different emotions might influence the allocation of AIA and help explain some of the behaviors associated with mood disorders, this current study should be considered preliminary. In addition to further testing of some of the hypotheses presented in this paper, more extensive research needs to control for the potential confounds and limitations of this study, including the small sample size used in this study. A larger number of subjects might increase power sufficiently to detect more subtle but significant differences. Additionally, the possibility remains that although the emotional experience was intense at the time the event took place, the intensity had substantially reduced at the time the memory was recalled. Studies using techniques such as functional imaging studies might better allow researchers to learn the parts of the brain that are activated by these emotional memories, and one of the goals of this report is to stimulate further research that will explore how experiencing an emotion can influence where individuals direct their action-intention and attention.
1. Sutton SK, Davidson RJ: Prefrontal brain asymmetry: a biological substrate of the behavioral approach and inhibition systems. Psychol Sci 1997; 8:204–210Google Scholar
2. Heilman KM: The neurobiology of emotional experience. J Neuropsychiatry 1997; 9:439–448Google Scholar
3. Lee GP, Loring DW, Meador KJ, et al: Hemispheric specialization for emotional expression: a reexamination of results from intracarotid administration of sodium amobarbital. Brain Cogn 1990; 12:267–280Google Scholar
4. Starkstein SE, Robinson RG: Mechanism of disinhibition after brain lesions. J Nerv Ment Dis 1997; 185:108–114Google Scholar
5. Lee GP, Meador KJ, Loring DW, et al: Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cogn Behav Neurol 2004; 17:9–17Google Scholar
6. Heilman KM, Van den Abell T: Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect). Neurology 1980; 30:327–330Google Scholar
7. Pardo JV, Fox PT, Raichle ME: Localization of a human system for sustained attention by positron emission tomography. Nature 1991; 349:61–64Google Scholar
8. Heilman KM, Chatterjee A, Doty LC: Hemispheric asymmetries of near-far spatial attention. Neuropsychology 1995; 9:58–61Google Scholar
9. Mishkin M, Ungerleider LG: Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res 1982; 6:57–77Google Scholar
10. Hillis AE, Newhart M, Heidler J, et al: Anatomy of spatial attention: insight from perfusion imaging and hemispatial neglect in acute stroke. J Neurosci 2005; 25:3161–3167Google Scholar
11. Rapcsak SZ, Cimino CR, Heilman KM: Altitudinal neglect. Neurology 1998; 38:277–281Google Scholar
12. Shelton PA, Bowers D, Heilman KM: Peripersonal and vertical neglect. Brain 1990; 113:191–205Google Scholar
13. Foster PS, Drago V, Webster DG, et al: Emotional influences on spatial attention. Neuropsychology 2008; 22:127–135Google Scholar
14. Foster PS, Harrison DW: The relationship between magnitude of cerebral activation and intensity of emotional arousal. Int J Neurosci 2002; 112:1463–1477Google Scholar
15. Lane RD, Reiman EM, Ahern GL, et al: Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 1997; 154:926–933Google Scholar
16. Markowitsch HJ, Vandekerckhove MM, Lanfermann H, et al: Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex 2003; 39:643–665Google Scholar
17. Reiman EM, Lane RD, Ahern GL, et al: Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry 1997; 154:918–925Google Scholar
18. Gilboa A: Autobiographical and episodic memory: one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia 2004; 42:1336–1349Google Scholar
19. Daselaar SM, Rice HJ, Greenberg DL, et al: The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cereb Cortex 2008; 18:217–229Google Scholar
20. Buchanan TW: Retrieval of emotional memories. Psychol Bull 2007; 133:761–779Google Scholar
21. Foster PS, Webster DG, Williamson JB: The psychophysiological differentiation of actual, imagined, and recollected mirth. Imagination, Cognition and Personality 2003; 22:163–180Google Scholar