Engaging in Cognitive Activities, Aging, and Mild Cognitive Impairment: A Population-Based Study
Abstract
The authors investigated whether engaging in cognitive activities is associated with aging and mild cognitive impairment (MCI) in a cross-sectional study derived from an ongoing population-based study of normal cognitive aging and MCI in Olmsted County, MN. A random sample of 1,321 study participants ages 70 to 89 (N=1,124 cognitively normal persons, and N=197 subjects with MCI) were interviewed about the frequency of cognitive activities carried out in late life (within 1 year of the date of interview). Computer activities; craft activities, such as knitting, quilting, etc.; playing games; and reading books were associated with decreased odds of having MCI. Social activities, such as traveling, were marginally significant. Even though the point-estimates for reading magazines, playing music, artistic activities, and group activities were associated with reduced odds of having MCI, none of these reached statistical significance. The equally high prevalence of reading newspapers in both groups yielded no significant between-group difference.
Mild cognitive impairment (MCI) is the intermediate stage between the cognitive changes of normal aging and those of dementia.1 Various sources can provide a detailed discussion of MCI.2,3 Subjects with MCI constitute a high-risk group because they develop dementia at a rate of 10% to 15% per year, as compared with 1% to 2% per year in the general population.4 In view of this, it is critical to identify potential protective factors against MCI. Previous studies have reported an association between cognitive activities and reduced risk of incident dementia.5–7 However, little is known about the association between cognitive activities and the odds of having MCI. A convenience sample of a prospective cohort study involving community-dwelling elderly participants reported that baseline cognitive activities were associated with decreased risk of amnestic MCI.8 There is a need to examine this question using a larger sample in a population-based setting.
We examined whether engaging in cognitive activities is associated with MCI in a cross-sectional study derived from an ongoing population-based study of normal cognitive aging and MCI in Olmsted County, MN. Throughout this manuscript, one can interchangeably think of the phrase “cognitive activity” to be equivalent to “mental activity” or “intellectual activity.”
METHOD
Setting
The detail of the design and conduct of the Mayo Clinic Study of Aging has been reported elsewhere.9 Briefly, it is an ongoing, population-based study of normal aging and MCI in Olmsted County, MN. Elderly persons ages 70 to 89 on the prevalence date of October 1, 2004, were recruited by using a stratified random sample from the target population of nearly 10,000 elderly individuals in Olmsted County. The sampling involved equal allocation of men and women in two age strata: 70–79 and 80–89 years old. During the first follow-up phase of the study, which took place in 2006 through 2008, we introduced a structured interview format to collect data on cognitive activities; 1,321 study participants without dementia completed the interview. At the time of the interview, neither the study participant nor the research personnel knew the case–control status of a participant. The classification of a study participant as having MCI or not was a downstream event to the collection of data on cognitive activities. The details of the classification process of MCI are discussed elsewhere in this article. The study was approved by the Institutional Review Boards of Mayo Clinic and the Olmsted Medical Center.
Measurement of MCI
The association of interest in this study is cognitive activities and odds of having MCI. Each participant in the Mayo Clinic Study of Aging underwent the following three face-to-face evaluations: 1) neurological evaluation by a physician; 2) risk-factor assessment by a nurse or study coordinator; and 3) neuropsychological testing that was interpreted by a neuropsychologist. The interview by the nurse or study coordinator included administration of the Clinical Dementia Rating Scale10 to the participant and to an informant. The neurological evaluation was performed by a physician and included administration of the Short Test of Mental Status,11 medical history review, and a complete neurological examination.
Neuropsychological testing was performed to assess four cognitive domains: 1) memory (Logical Memory–II [delayed recall] and Visual Reproduction–II [delayed recall] from the Wechsler Memory Scale–Revised (WMS–R), and Delayed Recall from the Auditory Verbal Learning Test);12–15 2) executive functioning (Trail-Making Test B,16 and Digit Symbol Substitution from the WAIS–R); 3) language (Boston Naming Test17 and Category Fluency;18) and 4) visuospatial skills (Picture Completion and Block Design from the WAIS–R).
We considered as Cases all the participants who met the revised Mayo Clinic criteria for MCI: 1) cognitive concern expressed by a physician, informant, participant, or nurse; 2) cognitive impairment in one or more domains (executive functioning, memory, language, or visuospatial); 3) normal functional activities; and 4) no dementia.2,3 Subjects with MCI could have a Clinical Dementia Rating Scale (CDR) score of 0 or 0.5; however, the final diagnosis of MCI was not based exclusively on the CDRS, but rather on all available data. The diagnosis of normal cognition, MCI, dementia, or Alzheimer's disease was made by an expert consensus panel of physicians, psychologists, and nurses on the basis of published criteria.2,9,19 The panel meets once per week and reviews three independent sources of data: 1) the clinical data collected by behavioral neurologists and physicians of other specialties with expertise in dementia and MCI; 2) neuropsychological data collected by psychometrists who are supervised by neuropsychologists; and 3) nursing data gathered by research nurses.9
Measurement of Cognitive Activities
We defined the exposure of interest to be reading, craft activities, computer activities, playing games, playing music, group activities (e.g., book club), social activities (e.g., going out to movies and theaters), artistic activities, and watching TV. We modified previously-validated instruments to measure these activities.6,22,23 A research nurse or psychometrist interviewed each participant by using a structured survey with ordinal responses (e.g., reading books at a frequency of once per week, twice per week, etc.). The participants were asked to provide information about these activities within 1 year of the date of interview (late-life cognitive activity). The measurement of cognitive activities was conducted along with neurological evaluation, neuropsychological assessment, and risk-factor ascertainments. Once these data were collected, then a consensus panel of experts classified the study participant to be cognitively normal or to have MCI.
Measurement of Covariates
In addition to traditional confounders (age, sex, and education), we also defined medical comorbidity and depression to be covariates for the purpose of this study. We measured medical comorbidity by using the Charlson Index, which is a widely used weighted index that takes into account the number and severity of diseases. Thus, for each unit-increase in Charlson Index, there is a stepwise increase in the cumulative mortality attributable to the comorbid medical disease.24 We measured depression by using the Beck Depression Inventory–II.25 Also, we adjusted for physical exercise by assigning a numeric score to frequency of physical exercise and adding the scores across the light, moderate, and vigorous strata (equal weighting to all strata). The details of the physical exercise measurement have been reported elsewhere.26
Statistical Analysis
We conducted multivariable logistic-regression analyses to examine the strength of association of cognitive activities with the odds of having MCI by computing odds ratios (ORs) and corresponding 95% confidence intervals (CIs). The primary analysis was conducted by adjusting for traditional confounders (age [continuous variable], sex, and education [continuous variable]). We also conducted secondary analysis by adjusting for medical comorbidity (weighted Charlson Index as a continuous variable), depressive symptoms (BDI–II score <13 versus ≥13), and physical exercise (continuous variable).26
The frequency of each activity was dichotomized as None (once per month or less) versus Any Other frequency. We considered watching TV to be hypothetically less beneficial; therefore watching TV was “reverse”-scored, that is, watching more TV (>6 hours/day) versus watching less (≤6 hours/day).
Analyses were conducted for cognitive activity carried out in late life (within the past year). Statistical testing was done at the conventional two-tailed α level of 0.05. All analyses were performed with SAS (Cary, NC).
RESULTS
Table 1 summarizes the demographic data. There were 1,321 study participants without dementia (N=1,124 cognitively normal persons, N=197 subjects with MCI). Among the cognitively normal group (Normals), there were an equal number of men and women, whereas, among the MCI group, there were more men than women. On average, the MCI group was older than the Normal group. The two groups also significantly differed in education, medical comorbidity, and depression symptoms. Therefore, in the primary analysis, the comparison of engaging in cognitive activities between the two groups was made after adjusting for age (continuous variable), sex, and education (continuous variable). In a secondary analysis, we also adjusted for depression symptoms, medical comorbidity, and level of physical exercise.
 |
Table 2 displays the data comparing the two groups as measured by OR (95% CI). Reading books (0.67 [0.49–0.94]), playing games (0.65 [0.47–0.90]), craft activities (quilting, pottery, etc.: (0.66 [0.47–0.93]), and computer activities (0.50 [0.36–0.71]) were significantly associated with decreased odds of having MCI. The point estimate for social activity (e.g., going out with friends) was also associated with decreased odds of having MCI, but this association was only marginally significant (0.71 [0.51–1.00]).
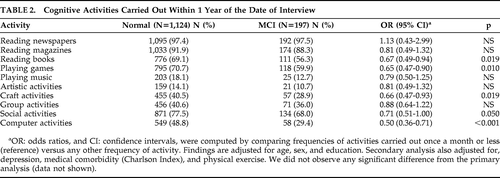 |
The point estimates for reading magazines (0.81 [0.49–1.32]), playing music (0.79 [0.50–1.25]), artistic activities (0.81 [0.49–1.32]), and group activities (0.88 [0.64–1.22]) were associated with reduced odds of MCI; however, none reached statistical significance. The only exception to the overall trend was the cognitive activity of reading newspapers. The OR for reading newspapers (1.13 [0.43–2.99]) suggested increased odds of having MCI; however, close examination of the data indicates that almost identical proportions of the two groups engaged in regular newspaper reading (97.4% of the cognitively normal group versus 97.5% of the MCI group were reading newspapers on a regular basis).
We considered watching TV to be a hypothetically less beneficial activity; therefore watching TV was “reverse”-scored, that is, watching more TV (>6 hours/day) versus watching less (≤6 hours per day). We observed that watching less TV was associated with decreased odds of having MCI (OR [95% CI]=(0.48 [0.27–0.86]; p=0.013].
In the secondary analysis, additional adjustment for depression symptoms, medical comorbidity, and physical exercise did not affect the significance level observed in the primary analysis (data not shown).
DISCUSSION
In this population-based, cross-sectional study, we observed that cognitive activities such as computer use, playing games, reading books, craft activities (quilting, knitting, etc.) and watching less TV were associated with 30% to 50% reduced odds of having MCI. Social activities such as traveling were marginally significant. Even though the point-estimates for reading magazines, playing music, artistic activities, and group activities were associated with reduced odds of having MCI, none reached statistical significance. Almost identical proportions of the two groups were engaged in reading newspapers on a regular basis; therefore we could not observe a significant difference between the two groups.
Several studies have reported the association of cognitive/intellectual or “mental” activities with decreased risk of incident dementia.5–7 However, little is known about the association of cognitive activities with MCI. The Bronx Aging Study prospectively followed a convenience sample of 437 community-dwelling cognitively normal elderly persons ages 75 and older to the outcome of incident amnestic MCI.8 During the median follow-up duration of 5.7 years, there were 58 subjects who developed incident amnestic MCI. The investigators noted that a unit-increase in cognitive activity was associated with a 5% decreased risk of incident amnestic MCI. Even though the Bronx study was a convenience sample, the prospective study design would enable one to make some degree of etiologic inferences. The investigators retrofitted the MCI criteria; hence, this might have potentially led to misclassification errors. Although our study is population-based, the cross-sectional design does not allow one to make etiologic inferences. Therefore, the observations made in our current study need to be tested on a larger sample in a prospective cohort design.
The findings of our study should be interpreted within the context of the following limitations: The first limitation pertains to study design. Since this was a cross-sectional study, we cannot determine the direction of causality between the hypothesized exposure of interest (i.e., cognitive activity) and the hypothesized outcome of interest (i.e., MCI). Second, like any survey-based study, recall bias is a potential limitation. This is even more relevant to participants with MCI; however, at our center, the data on cognitive activities are collected before determination of whether a person has MCI. Therefore, neither the participant nor the research personnel knew the case–control status of the participant at the time of administration of the cognitive-activities questionnaire. This likely minimized bias, but could not eliminate it. Also, in the past, we had reported that the test–retest correlations were similar among subjects with normal cognition and MCI.26
Our study did not address mechanism of action. However, the possible beneficial impact of cognitive activities as discussed in the literature is worth mentioning. Engaging in cognitive activities may be a marker for an overall healthy lifestyle; for example, a person who likes to read books on a regular basis may also engage in an overall healthy lifestyle that includes exercise, diet, and stress-management. Another possible explanation is related to the brain/cognitive-reserve hypothesis.27,28 Engaging in cognitive activity is more likely to reinforce and perhaps stimulate the formation of various neuronal networks in the brain28 that can buffer against dementia and Alzheimer's disease (AD).29 This argument is supported by both basic science and clinical research. For instance, animals with enriched environments are protected against cognitive impairment.28,30 Also, in clinical settings, it is observed that clinical manifestations may not correlate with the neuropathological burden on postmortem examination,6,31–33 which implies that the cognitive reserve may serve as a buffer against the AD neuropathological burden. Since MCI is considered to be a prodromal state to AD, one can invoke the cognitive-reserve theory to explain the inverse association between cognitive activities and the odds of having MCI. Yet another potential mechanism pertains to the classic stress model proposed by Sapolsky and colleagues.34 According to this model, the hippocampus, which is the epicenter of the memory network,35 has a number of glucocorticoid receptors. These receptors are down-regulated in excessively stressful situations. Thus, cognitive activities may serve as stress-modifying agents, leading to decreased “neurotoxic” insult to the hippocampus and related structures pertinent to cognition and emotion.
In summary, our findings contribute to the growing body of literature indicating that cognitive activities are associated with decreased odds of having MCI. A future prospective, population-based cohort study needs to confirm whether cognitive activity is associated with a decreased risk of incident MCI. We are following a large cohort of cognitively normal persons for the outcome of incident MCI; thus, we will be able to test the observation made from the current cross-sectional study. The population-based setting will improve generalizability, and the prospective cohort will strengthen etiologic inferences.
1. : Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005; 352:2379–2388Crossref, Medline, Google Scholar
2. : Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256:183–194Crossref, Medline, Google Scholar
3. : Mild cognitive impairment: beyond controversies, toward a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256:240–246Crossref, Medline, Google Scholar
4. : Current concepts in mild cognitive impairment. Arch Neurol 2001; 58:1985–1992Crossref, Medline, Google Scholar
5. : An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 2004; 3:343–353Crossref, Medline, Google Scholar
6. : Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003; 348:2508–2516Crossref, Medline, Google Scholar
7. : Participation in cognitively-stimulating activities and risk of incident Alzheimer disease. JAMA 2002; 287:742–748Crossref, Medline, Google Scholar
8. : Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology 2006; 66:821–827Crossref, Medline, Google Scholar
9. : The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures, and sample characteristics. Neuroepidemiology 2008; 30:58–69Crossref, Medline, Google Scholar
10. : The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414Crossref, Medline, Google Scholar
11. : The Short Test of Mental Status: correlations with standardized psychometric testing. Arch Neurol 1991; 48:725–728Crossref, Medline, Google Scholar
12. : Mayo's Older Americans Normative Studies: WAIS–R norms for ages 56 to 97. Clin Neuropsychol 1992; 6(suppl):1–30Crossref, Google Scholar
13. : Mayo's Older Americans Normative Studies: WMS–R norms for ages 56 to 94. Clin Neuropsychol 1992; 6(suppl):49–82Crossref, Google Scholar
14. : Mayo's Older Americans Normative Studies: updated AVLT norms for ages 56 through 97. Clin Neuropsychol 1992; 6(suppl):83–104Crossref, Google Scholar
15. : Mayo's Older Americans Normative Studies: utility of corrections for age and education for the WAIS–R. Clin Neuropsychol 1992; 6(suppl):31–47Crossref, Google Scholar
16. : Validity of the Trail-Making Test as an indicator of organic brain damage. Percep Mot Skills 1958; 8:271–276Crossref, Google Scholar
17. . The Boston Naming Test, 2nd Ed. Philadelphia, PA, Lea & Febiger, 1982Google Scholar
18. : Mayo Older American Studies: category fluency norms. J Clin Exp Neuropsychol 1998; 20:194–200Crossref, Medline, Google Scholar
19.
20. : Mayo's Older Americans Normative Studies: WAIS–R, WMS–R, and AVLT norms for ages 56 through 97. Clin Neuropsychol 1992; 6(suppl.):1–104Crossref, Google Scholar
21. : Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308Crossref, Medline, Google Scholar
22. : Cognitive activity in older persons from a geographically-defined population. J Gerontol B Psychol Sci Soc Sci 1999; 54(3):P155–P160Crossref, Medline, Google Scholar
23. : Patients with Alzheimer's disease have reduced activities in midlife compared with healthy control-group members. Proc Natl Acad Sci U S A 2001; 98:3440–3445Crossref, Medline, Google Scholar
24. : A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383Crossref, Medline, Google Scholar
25. : Manual for Beck Depression Inventory–II (BDI–II). San Antonio, TX, Psychology Corporation, 2001Google Scholar
26. : Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol 2010; 67:80–86Crossref, Medline, Google Scholar
27. : Education and the prevalence of dementia and Alzheimer's disease. Neurology 1993; 43:13–20Crossref, Medline, Google Scholar
28. : What is cognitive reserve? theory and research application of the reserve concept. J Int Neuropsychol Soc 2002; 8:448–460Crossref, Medline, Google Scholar
29. : Linguistic ability in early life and cognitive function and Alzheimer's disease in late life: findings from The Nun Study. JAMA 1996; 275:528–532Crossref, Medline, Google Scholar
30. : Neural consequences of environmental enrichment. Nat Rev Neurosci 2000; 1:191–198Crossref, Medline, Google Scholar
31. : Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006; 66:1837–1844Crossref, Medline, Google Scholar
32. : Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol 1999; 58:376–388Crossref, Medline, Google Scholar
33. : Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol 2008; 65:1211–1217Crossref, Medline, Google Scholar
34. : Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol 1999; 34:721–732Crossref, Medline, Google Scholar
35. : From sensation to cognition. Brain 1998; 121:1013–1052Crossref, Medline, Google Scholar



