Cortical Interactions During the Experience of Auditory Verbal Hallucinations
Abstract
Auditory verbal hallucinations (AVHs), the perception of voices in the absence of auditory stimuli, are common and distressing symptoms reported by 50%–80% of patients with schizophrenia. However, the results in a number of imaging and electrophysiological studies on the origins of AVH are not consistent, and the underlying pathophysiology remains unclear. The authors enrolled a group of schizophrenia patients and normal-control subjects, age 18–45 years. Two patient groups participated in the study; 1) a group of 8 patients with drug-resistant spontaneous AVHs; and 2) a group of 7 patients whose AVHs were successfully controlled with neuroleptic medication; along with 16 normal-control subjects. The entire sample had EEG recording done, with the AVH group told to press a button when they experienced a hallucination, and the other two groups randomly told when to press the button. In the AVH group, hallucinations were longer in the “eyes-closed” than “eyes-open” condition. There was spreading phase-coupling in the AVH group, intra- and inter-hemispherically, at left and right frontal and temporal areas, under both eyes-closed and eyes-open condition, during the experience of AVH. There was a statistically significant increase of α-band frequency-specific synchrony maximum values in the AVH group. AVHs are considered to be complex features, and, as such, they reflect abnormal functional connectivity in multiple related regions in both intra- and inter-hemispherical brain sites, primarily defined by phase-integration.
Auditory verbal hallucinations (AVHs), the perception of voices in the absence of auditory stimuli, are common and distressing symptoms reported by 50%–80% of patients with schizophrenia.1 However, the results in a number of imaging and electrophysiological studies on the origins of AVH, are not consistent, and the underlying pathophysiology still remains unclear. Neuroimaging studies have associated occurrences of AVHs with activation of diverse brain regions involved in speech generation, speech perception, and verbal memory.2–7 Many of these studies have found prominent activation during AVHs in the right as well as left hemisphere,4,5 with considerable intersubject variation in the cortical areas involved.6
A “symptom-capture” approach attempts to explain AVHs by imaging the dynamic changes of brain functioning in terms of EEG activity, blood flow, and metabolism, targeting the “envelope of the symptom,” using EEG, functional MRI (fMRI), or positron emission tomography (PET), in periods associated with the appearance of auditory hallucinations. This approach was first introduced in 19952 as a method for capturing hallucinations during [15]O PET scanning. Although this approach is conceptually simple, a number of confounding factors related to the ability of the patient to precisely report the initiation and completion of the hallucinatory experience affect the temporal location of the hallucination. Nevertheless, a number of investigators have used this approach successfully, reporting that auditory hallucinations are associated with activation of speech-production areas, primary and secondary auditory cortices, and various polymodal association cortices.3,5 From an electrophysiological point of view, limited research data have given support to the central auditory-processing deficit model. These studies showed that AVHs are associated with increased beta-frequency oscillations generated in speech-related areas.8 Moreover, increased α-band relative coherence between the left and right superior temporal cortices has been reported during AVHs.9
The aim of the present study was to detect possible changes of EEG properties temporally anchored in the vicinity of the hallucinatory experience in schizophrenic patients with persistent AVHs. Thus, we exclusively focused on the brain functioning associated with the experience of AVHs and did not extend the focus to a description of global or state EEG characteristics related to schizophrenia in general. Under this experimental design, the EEG phase-stability of selected brain sites was considered as an expression of the functional coupling and was assessed in the presence of AVHs. Two different computations of phase synchrony were applied in our EEG recordings: 1) the band-specific synchrony (BSS), which expresses the spatial-temporal average of the instantaneous neuronal coupling; and 2) the frequency-specific synchrony (FSS), which focuses on the temporal mode of the synchrony associated with the experience of AVHs, as reported by the patients.
The study compared two patient groups suffering from schizophrenia: 1) patients with auditory verbal hallucinations; and 2) patients without hallucinations; with a third group of normal subjects. The authors examined BSS and FSS in the broad frequency region of α EEG band (6–13 Hz).
METHOD
A group of 17 schizophrenia patients and 16 normal-control subjects participated in the study. After a detailed description of the experimental protocol, all subjects gave written informed consent, and we obtained University Mental Health Research Institute Ethics Committee approval. The inclusion criteria were the following: 1) age between 18 and 45 years; 2) right-handed; 3) no alcohol and drug abuse in the last 5 years; 4) no neurological illness or head trauma that would result in an abnormal EEG; 5) no antiepileptic drugs; and 6) no alcohol use in the last 24 hours. Normal-controls had no Axis I psychiatric disorder. Two patients' data were excluded from the analysis because of artifacts related to limited cooperation during the experimental procedure. All patients were recruited from Eginition University Hospital and fulfilled the criteria for schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition–Text Revision (DSM-IV–TR; 2000). The Positive and Negative Symptoms Scale (PANSS) was used for assessment of symptoms. The detailed characteristics of the hallucinations were assessed with the Psychotic Symptom Rating Scales–Auditory Hallucinations Rating Scale (PSYRATS–AHRS).10 All clinical data were collected 1 day before the EEG recording.
Two groups of patients participated in the study; first: the SCZ-AVHs group consisted of 8 patients with drug-resistant spontaneous AVHs (4 men, 4 women; mean age: 36 (SD: 7) years; duration of illness: 15.5 (SD: 6) years; mean PANSS score: 70 (SD: 6); 6 of these patients were medicated with risperidone (mean dose: 7 mg/day) and 2 with amisulpride (mean dose: 800 mg/day); and second: the SCZ group, consisting of 7 patients who, at the time of enrollment, did not exhibit AVHs, as a result of effective antipsychotic drug treatment, (3 men, 4 women, mean age: 30 (SD: 9); duration of illness: 13 (SD: 8) years; mean PANSS score: 68 (SD: 8). Four of these patients were medicated with risperidone (mean dose: 7 mg/day), two with amisulpride (mean dose: 600 mg/day), and one with aripiprazole (dose: 25 mg/day). All participants were selected from a larger sample of subjects suffering from schizophrenia, but who had the ability to cooperate in the experimental procedure. The Control (NOR) group consisted of 16 subjects (8 men, 8 women); mean age: 31 (SD: 6) years.
Subjects were seated in a light- and sound-attenuated, double-skin Faraday cage. Electrodes (Fp1, Fp2, F7, F3, Fz, F4, F8, FT7, FC3, FCz, FC4, FT8, T7, C3, Cz, C4, T8, TP7, CP3, CPz, CP4, TP8, P7, P3, Pz, P4, P8, O1, Oz, O2) were placed on the scalp, using a standard cap. Recordings of the horizontal-plane eye-movement potentials were made by two electrodes fixed 1 cm bilateral to the outer canthus of each eye. The skin resistance of each electrode was kept at ≤5 kΩ for the entire session. The participants were instructed to report any experience of auditory hallucination. This was achieved by pressing a miniature optical switch with the middle finger of their dominant hand to indicate the onset and the duration of any experienced AVH. In order to validate our findings, the data obtained from the SCZ-AVHs group were compared with those obtained by the NOR and SCZ, where the participants were instructed to press the button in a voluntary basis, without any previous prompt. The above procedure was applied both during eyes-open (E-OP) and eyes-closed (E-CL) conditions. The EEG signals were acquired by a Synamps (Neuroscan Labs) amplifier module sampled at 500 Hz. The phase stability of selected sites was computed by the following equation: 
RESULTS
A total number of 472 hallucinations were reported by the SCZ-AVHs patients during the recording procedure. The mean duration of hallucinatory experience was 8.1 (SD: 9) sec under the E-CL and 5.3 (SD: 6.5) sec under the E-OP experimental condition. The rate of acousmata (hallucinations/min) under the E-CL condition (mean: 6.62 (SD: 1.9) was found to be significantly higher than the rate of acousmata in the E-OP condition (mean: 4.1 [SD: 2]); ANOVA (F[1,14]=4.73; p<0.05). Time-averaged BSS computed from EEG activity in SCZ-AVHs, SCZ, and NOR subjects (mapped in Figure 1) specifically showed spreading phase-coupling in the SCZ-AVHs group, intra- and inter-hemispherically, at left and right frontal and temporal areas under both eyes-closed and eyes-open condition, with this finding more pronounced under the eyes-closed condition. The BSS distribution of the NOR as well as the SCZ group, under the same experimental condition, did not show persistent synchrony. In this manner, BSS results showed the general tendency to brain coupling during periods with sustained AVHs, independently of the moment that the hallucinatory events occurred.
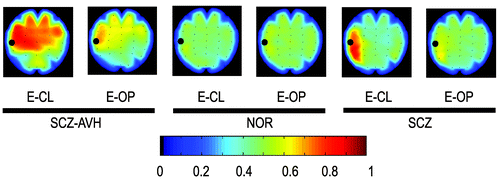
Black circles denote the reference electrode for the specific map. The color reflects the average strength of phase coupling.
Following the “symptom-capture” approach, we attempted to relate AVH experiences with synchrony fluctuations before and after the end of the AVH w1 and w2 windows respectively (see Figure 2 [a]). As shown in Figure 2 [a], the synchrony traces converged during the AVH, while they diffused after the end of the experience (blue vertical bar). The encircled cluster of traces includes the synchrony of the anterior part of the brain of both hemispheres with respect to the T7 electrode. The distribution of synchrony values regarding the w1 and w2 time windows was used as a measure to characterize the dynamic mode of coupling observed in the frontal brain areas during an AVH event. The observed shift of synchrony distribution to higher values before and lower values after the end of an AVH, was a common finding in the majority of the cases examined (Figure 2 [b], [c]). In the above-illustrated case, the underlying oscillatory activity recorded at T7 and T8 showed increased amplitude, accompanied with high synchrony envelopes, before the specific AVH, indicating strong inter-hemispheric coupling (Figure 2 [d]–[f]).
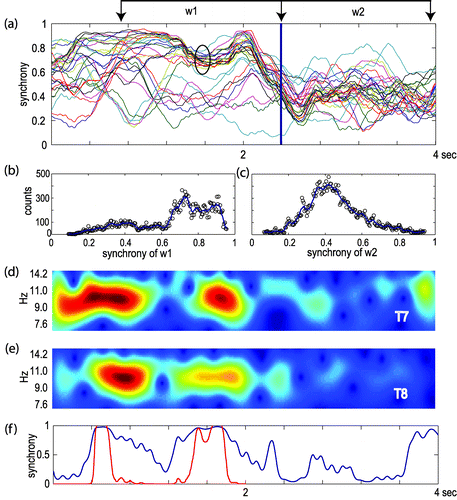
[a] α-band synchrony (BSS) of a 4-sec EEG epoch. Each line of the graph represents the computed synchrony of one recorded site in respect to T7 electrode. The circled lines correspond to the anterior part of the brain of both hemispheres (FT7, F7, Fp1, C3, FC3, F3, Cz, FCz, Fz, T8, FT8, F8, Fp2, C4, FC4, F4). Vertical blue line indicates the end of an AVH experience. [b] and [c] the distribution of synchrony, expressed as counts per synchrony bin, observed in two 1.5-sec windows before and after the AVH notification, w1 and w2, respectively. [d] and [e] energy envelopes of the oscillation recorded at T7 and T8 electrode sites. [f] coupling vs. time in the frequency bin (9.1 Hz) where synchrony exhibits the maximum. Blue line shows the instantaneous FSS. Red line shows the comparison of the instantaneous FSS with the distribution of the surrogate data-set. Plots [a] to [f] refer to the same 4-sec. EEG segment.
The authors subjected to statistical analysis 40 synchrony cases obtained from SCZ-AVHs in whom the inter-hallucination interval was greater than 8 sec, with 40 cases (“dummy” pressings) of SCZ and NOR groups. In the SCZ-AVHs group, the maximum synchrony values were estimated within a 1.5-sec window before the end of the hallucinations as these were denoted by the subjects. In the same way, the maximum synchrony values of the SCZ and NOR groups were estimated with respect to the timing of dummy pressings. Maximum synchrony values of the SCZ-AVHs group (mean: 0.63; SD: 0.7) were found to be significantly greater than those of the NOR (mean: 0.29; SD: 0.19) as well as the SCZ group (mean: 0.26; SD: 0.25; ANOVA (F[1,78]=69.23; p<0.001; and F[1,78]=56.55; p<0.001), respectively. No differences were found between the SCZ and NOR groups ANOVA (F[1,78]=0.43; NS).
Likewise, significantly longer latency values between the button-release and the maximum synchrony were found between the SCZ-AVHs group (mean latency: 760 [SD: 293] msec) and the NOR (mean latency: 450 [SD: 411] msec) as well as the SCZ (mean latency: 453 [SD: 358] msec) group; (ANOVA: F[1,78]=7.95; p<0.01) and (F[1,78]=142.72; p<0.01), respectively. No differences were found between the SCZ and NOR groups (F[1, 78]=0.014; NS). The above findings indicate that concentration of synchrony peaks converged in a limited time-window before the end of the button-pressing in the case of SCZ-AVH group. In the SCZ and NOR groups, the absolute values of the observed synchrony peaks, as well as the latencies, were widely distributed, suggesting that these peaks were not causally related.
DISCUSSION
Spontaneous EEG oscillations at various frequencies exhibit transient phase-concordance, which systematically relates to behavioral and or experimental conditions. These EEG oscillations seem to play an important role in the spatial characteristics of the neuronal assemblies involved in specific processes,13 and their extent provides information regarding the neuro-cognitive processes involved in specific psychiatric symptoms such as AVHs.
The evidence that α-band activity is the most pronounced EEG oscillation between the temporal regions bilaterally,14 along with our finding in which a significant increase in the frequency of AVHs has been observed during the eyes-closed, versus the eyes-open, condition raised the question of possible involvement of α oscillations in the inception of a neurocognitive state in which the production of AVHs can be experienced by the subject. This led us to focus on analysis of the phasic characteristics of a broad α EEG frequency region in the auditory-related cortices of hallucinatory patients. In this line of evidence, our results further contribute to the understanding the specific role of α oscillations recently reported in 2001 and 2007, respectively.15,16 In the present study, we found 1) An increased rate of AVHs experienced by the SCH-AVH subjects during the E-CL, as compared with the E-OP experimental condition; 2). An increased phase-coupling of α-band (BSS analysis) in the SCZ-AVH group distributed intra- and inter-hemispherically in the anterior brain areas under both E-CL and E-OP conditions; 3) This was more profound under the E-CL condition; 4) A statistically significant increase of α-band FSS maximum synchrony values in the SCZ-AVHs group, as compared with the NOR and the SCZ groups at the T7–T8 electrode pair inter-hemispherically.
These synchrony values were observed in a time-window related to the report of the hallucinatory experience. In the same way, significant difference in latency was found between the SCZ-AVHs group and the SCZ/NOR groups between the AVH report and the peak of synchrony observed. These results support the assumption of an α oscillation involvement, by means of phase-coupling, in the processes underlying the production of AVHs. In α-band amplitude studies, α oscillations have been thought to reflect idling17 or active inhibition of task-irrelevant brain circuits.18,19 However, recent data on α amplitude and, in particular, α phase-dynamics, posit a direct and active role for α-band rhythmicity in the mechanisms of attention and consciousness. This view is supported by the positive correlation of the α amplitude with short-term memory and working-memory load18,19 and task difficulty.20 Furthermore, α oscillations can phase-lock between widely-separated cortical regions21,22 and, thus, form functional large-scale networks.23
Enhanced α-band synchrony in the fronto-parietal network is associated with the execution of cognitive tasks.22 However, in studies reporting strengthened α-phase synchrony, some show a simultaneous amplitude increase,24 whereas others show an associated amplitude suppression.22,25 In our study, high-amplitude α oscillations were associated with long-distance phase-coupling in time-windows closely related to the hallucinatory experiences in the symptom-capture paradigm. Our results illustrate increased intra- and inter-hemispheric temporal coupling during persistent hallucinatory states. This finding is in agreement with observations made in 2005 in an EEG coherence study,9 where an abnormal increase of the inter-hemispheric functional connections between the auditory cortices was observed during the experience of AVHs.
During phase computations, the contribution of a volume-conduction component should be carefully considered, particularly between the proximal electrode sites. In this study, the long-distance synchronization found excludes, by definition, the influence of this factor. Moreover, in the FSS analysis, the synchrony peaks found were temporally engaged with the marked AVHs, a fact that reduces the effect of phase leak due to the volume conductance. Another issue that should be considered in our methodology is the confounding factors related to the ability of the patient to precisely report the initiation and completion of the hallucinatory experience. However, in order to limit subjectivity errors, the authors carefully selected patients with insight, high level of education, and good social functioning, to improve the cooperation during the experimental procedure. Auditory verbal hallucinations are considered to be complex features, and, as such, they reflect abnormal functional connectivity in multiple related regions in both intra- and inter-hemispherical sites. Since brain functional connectivity is defined primarily by phase-integration; our findings indicate that acousmata are related to abnormal neuronal synchrony that implies erroneous and, in a sense, autonomous functional connectivity.
1. : The auditory hallucination: a phenomenological survey. Psychol Med 1996; 26:177–189Crossref, Medline, Google Scholar
2. : A functional neuroanatomy of hallucinations in schizophrenia. Nature 1995; 378:176–179Crossref, Medline, Google Scholar
3. : Activation of Heschl's gyrus during auditory hallucinations. Neuron 1999; 22:615–621Crossref, Medline, Google Scholar
4. : Spatial and temporal mapping of neural activity associated with auditory hallucinations. Lancet 1999; 353:644Crossref, Medline, Google Scholar
5. : Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 2000; 57:1033–1038Crossref, Medline, Google Scholar
6. : Cortical activation associated with the experience of auditory hallucinations and perception of human speech in schizophrenia: a PET correlation study. Psychiatry Res 2003; 122:139–152Crossref, Medline, Google Scholar
7. : The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. Neuroimage 2005; 27:644–655Crossref, Medline, Google Scholar
8. : Quantitative EEG and low-resolution electromagnetic tomography (LORETA) imaging of patients with persistent auditory hallucinations. Schizophr Res 2006; 83:111–119Crossref, Medline, Google Scholar
9. : EEG coherence measures during auditory hallucinations in schizophrenia. Psychiatry Res 2005; 136:189–200Crossref, Medline, Google Scholar
10. : Scales to measure dimensions of hallucinations and delusions: The Psychotic Symptom Rating Scales (PSYRATS). Psychol Med 1999; 29:879–889Crossref, Medline, Google Scholar
11. : Studying single trials of phase-sychronous activity in the brain. Int J Bifurcat Chaos 2000; 10:2429–2439Crossref, Google Scholar
12. : Phase synchronization: from theory to data analysis, in Neuro-Informatics and Neural Modelling. Edited by Moss FGielden S. Amsterdam, The Netherlands, Elsevier, 2000, pp 279–321Google Scholar
13. : The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull 2008; 34:927–943Crossref, Medline, Google Scholar
14. : Detection of very high correlation in the alpha band between temporal regions of the human brain using MEG. NeuroImage 2002; 22:1432–1437Crossref, Google Scholar
15. : Spatial-temporal structures of human alpha rhythms: theory microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp 2001; 13:125–164Crossref, Medline, Google Scholar
16. : New vistas for α-frequency band oscillations. Trends Neurosci 2007; 30:150–158Crossref, Medline, Google Scholar
17. : Event-related synchronization (ERS) in the alpha band: an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol 1996; 24:39–46Crossref, Medline, Google Scholar
18. : Object-load and feature-load modulate EEG in a short-term memory task. Neuroreport 2003; 14:1721–1724Crossref, Medline, Google Scholar
19. : Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex 2002; 12:877–882Crossref, Medline, Google Scholar
20. : EEG alpha synchronization and functional coupling during top-down processing in a working memory task. Hum Brain Mapp 2005; 26:148–155Crossref, Medline, Google Scholar
21. : Top-down processing mediated by inter-areal synchronization. Proc Natl Acad Sci 2000; 97:14748–14753Crossref, Medline, Google Scholar
22. : Rapid distributed fronto-parieto-occipital processing stages during working memory in humans. Cereb Cortex 2002; 12:710–728Crossref, Medline, Google Scholar
23. : The brainweb: phase-synchronization and large-scale integration. Nat Rev Neurosci 2001; 2:229–239Crossref, Medline, Google Scholar
24. : Phase-synchrony among neuronal oscillations in the human cortex. J Neurosci 2005; 25:3962–3972Crossref, Medline, Google Scholar
25. : Transient inter-hemispheric neuronal synchrony correlates with object recognition. J Neurosci 2001; 21:3942–3948Crossref, Medline, Google Scholar



