Chronic Alcohol Use and Sleep Homeostasis: Risk Factors and Neuroimaging of Recovery
Alcohol use disorders are among the most prevalent and commonly comorbid mental health conditions. Chronic alcohol use is associated with many behavioral concerns, including complaints with sleep in 35%−91% of patients.16,17 The most common problems include increased sleep latency (i.e., difficulty initiating sleep), poor sleep quality, and daytime sleepiness. Often, these sleep problems will persist through withdrawal but are expected to resolve following prolonged abstinence.18 The effects of alcohol on sleep have detrimental socioeconomic consequences, as alcohol associated sleep problems account for approximately 10% of the annual costs related to alcohol use disorders.19,20 The DSM-5 defines alcohol use disorder as a problematic pattern of alcohol use that leads to the presence of at least two pervasive social (e.g., neglecting social functions), functional (e.g., use when physically hazardous), or health (e.g., tolerance and/or withdrawal) impairments/symptoms that occur within a one year time span.21 This can differ from long-term heavy alcohol use that is present, but not problematic. Research studies vary considerably in their criteria for alcohol use and abuse, so the collective term “chronic alcohol use” will be utilized.
The normal mechanisms of sleep-wake cycling are largely regulated by a balance of homeostatic drive promoting sleep integrated with the circadian system that maintains timing of wakefulness and sleep in the day-night cycle (Figure 1).1,2,4,5,22–24 The sleep-wake circadian rhythm is a biological process sustaining an endogenous oscillation of approximately 24 hours when driven by light and darkness in the environment. Sleep and circadian rhythms are bidirectionally linked and have substantial overlap between and integration of underlying neurobiological systems.22,25 The suprachiasmatic nucleus in the anterior hypothalamus is the master circadian clock in mammals.26 Circadian rhythms generated by the suprachiasmatic nucleus are entrained to the daily light-dark cycle. Daily phase resetting is mediated by input from specialized (melanopsin containing) retinal ganglion cells. There is also extensive overlap between the circadian clock and stress response systems, such that the hypothalamic-pituitary-adrenal axis and glucocorticoid systems are temporally controlled by the circadian clock.27
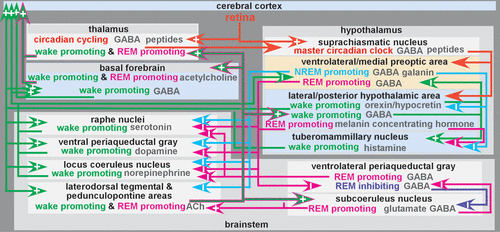
FIGURE 1. The sleep-wake cycle is presently conceptualized as a two-process system, with summation of circadian and sleep-inducing influences governing state of arousal dynamically.1–5 Diagrammed is a simplified presentation of major structures participating in the sleep-wake cycle and important interconnections, color-coded by major actions: circadian (red), wake-promoting ascending arousal system (green), NREM sleep promoting (blue), REM sleep promoting (pink), REM sleep inhibiting (purple. Sleep-promoting actions of extracellular adenosine are color-coded by direction of action: sleep-promoting inhibition (light blue background), sleep promoting excitation (light gold background). Note that increased release of GABA from the hypothalamic ventrolateral/medial preoptic area projections inhibits wake-promoting areas, ultimately inducing NREM sleep.
Sleep drive gradually intensifies during wake time and dissipates during sleep. One contributor to sleep drive is buildup of sleep modulating substances (i.e., sleep factors) during the waking period in multiple brain regions.2,28 Several sleep factors have been identified (e.g., adenosine, nitric oxide, tumor necrosis factor alpha, interleukin 1).2,5,20 Adenosine has been of particular interest because its formation is related to neuronal activity and caffeine is an adenosine A1 receptor antagonist.2,5,20 It also may serve as a final common pathway for other sleep factors.5,20 Adenosine accumulation promotes sleep by inhibiting (via A1 receptors) wake-promoting neurons in the basal forebrain and lateral hypothalamus and by exciting (via A2 receptors) sleep-promoting neurons in the ventrolateral preoptic area of the hypothalamus (Figure 1).5,20,29,30 This wakefulness-dependent increase in adenosine peaks before sleep onset. It is thought to promote sleep onset by inhibiting ascending arousal-related projections of the brainstem and basal forebrain regions (Figure 1). During daylight hours, circadian arousal-promoting mechanisms counter the sleep drive to maintain wakefulness. This influence subsides as evening approaches and sleep drive peaks. Nonrapid eye movement (NREM) sleep early in the sleep period will be greater with higher adenosine accumulation. Adenosine will dissipate throughout the sleep cycle as rapid eye movement (REM) sleep commences.31,32 Within the homeostatic sleep drive, an ultradian rhythm regulates the approximate 90-minute cyclical alteration of NREM and REM sleep.33
Preclinical studies have provided some insights into ways that alcohol may interact with components of the sleep-wake system. Adenosine is implicated in the pathophysiology of both sleep disorders and chronic alcohol use.34 Alcohol administration induces an increase in extracellular adenosine level in some brain regions, resulting in increased inhibition.35 Studies in cultured cells indicate that acute alcohol exposure inhibits transporter mediated (equilibrative nucleoside transporter 1, ENT1) reuptake of adenosine. Research with rodents suggests that alcohol induces dose-dependent adenosine accumulation in the basal forebrain that inhibits wake-promoting neurons.36 Downregulation of both ENT1 and A1 receptor expression in the basal forebrain has been demonstrated during acute withdrawal following development of alcohol dependency.35,37,38 As noted by these authors, insomnia during withdrawal may be due in part to reduced inhibition of basal forebrain wake-promoting neurons disrupting sleep homeostasis. Acute administration of alcohol enhances inhibition by both increasing GABA activity (e.g., activating receptors, facilitating release) and decreasing glutamate activity (e.g., inactivating receptors), likely mediating some of the sedative properties.17,19 It has been suggested that a primary causal mechanism of increased NREM sleep following acute alcohol consumption is the inhibition of wake-promoting neurons through activation of GABAA receptors. Decreased REM sleep may be due to the activation of GABAB receptors and/or inhibition of kainate receptors on brainstem cholinergic cells.19 Chronic exposure to high doses of alcohol induces adaptive changes (e.g., decreased inhibitory response of GABAA receptor systems, increased activity of excitatory glutamate receptor systems), which may contribute to tolerance to the sedative effects.17,19 In addition, six weeks of chronic exposure to alcohol is sufficient to induce desynchrony among circadian rhythms (e.g., core body temperature, activity level, hormonal secretion) and phase shift of peripheral circadian clocks.39
In laboratory studies, ingestion of a moderate amount of alcohol before bedtime in healthy individuals is typically associated with decreased sleep latency, increased NREM sleep, increased total sleep time, and reduced or fragmented REM sleep that leads to decreased sleep efficiency.17,19,20,40,41 This is followed by rebound increases of REM sleep on subsequent nights.42,43 Similarly, a longitudinal community study (months) that utilized multilevel modeling to examine associations between daily alcohol use and each night’s sleep found a positive association with sleep duration and a negative association with sleep quality.44 Of note, the range for within-person association was wide, indicating considerable individual differences to the effects of alcohol use. It has been suggested that tolerance to the sleep-enhancing effects of alcohol can develop quickly (e.g., 3 to 9 nights of daily use), although this likely varies by person.17,45 Alcohol use before bedtime can also disrupt circadian rhythms.25,46 Decreased melatonin secretion and altered core body temperature rhythm are the most frequently reported effects.25
Sustained chronic abuse of alcohol may result in detrimental changes in multiple aspects of sleep. Chronic alcohol use is associated with longer sleep latency, altered NREM sleep, decreased and disrupted REM sleep, and reduced total sleep time.19,41,47 These changes occur as individuals who use alcohol chronically become tolerant to the sleep-enhancing effects of alcohol, but remain sensitive to the stimulating effects.19,48 During periods of heavier drinking (e.g., binge drinking), chronic users may experience reduced sleep latency and increased NREM sleep, similar to acute effects of alcohol in healthy individuals.48 Sleep duration is still typically shortened overall. Although there is limited research on changes to circadian rhythms associated with chronic alcohol use, the most commonly reported finding is altered melatonin secretion (e.g., elevated daytime levels, delayed phase).25,46,49 Based on the known interactions among these systems, it is likely that the hypothalamic-pituitary-adrenal axis is involved in mediating some of the negative effects of chronic alcohol use on sleep and circadian rhythms.39
Most human studies evaluating the effects of chronic use of alcohol on sleep focus on acute and postacute withdrawal. During acute withdrawal, sleep latency increases and total sleep decreases (relative to drinking periods), congruent with the development of tolerance to the hypnotic effects of alcohol.16,19 Acute withdrawal is also associated with a decrease in NREM sleep, more frequent REM episodes, reduction of REM latency, and shortened NREM-REM cycles, but without increased total REM duration.47,50 Higher REM sleep deficit is typically seen in abstinent individuals compared with healthy controls, and it has been suggested that this may be both a pre-existing risk factor for chronic alcohol use as well as for relapse.17 Although NREM sleep will improve over time abstinent, reports are varied as to how and when specific stages of NREM sleep stabilize.16,45,51 Early research suggested that stage one NREM sleep stabilizes after a year, stage three within several months, and reports of stage four sleep are variable (use of the term stage four sleep has been discontinued due to the unclear distinction between sleep stages three and four).16,52 Altered melatonin phase timing appears to normalize with abstinence, although it may be worsened temporarily during acute withdrawal.25,49,53,54 Persistent sleep problems are marked by reduced amounts of NREM sleep and shortened sleep times overall with prolonged disorganization of circadian rhythms, the homeostatic sleep system, and disruptions of transitions between sleep stages.16,25
Autopsy studies have reported that uncomplicated chronic alcohol use is associated with mild brain atrophy, primarily due to decreased white matter, with clear evidence of cellular loss only in lateral prefrontal cortex.6,7 In contrast, magnetic resonance imaging (MRI) studies have reported both decreases in white matter and widespread areas of cortical thinning and/or reduced gray matter volume.6,7 Two meta-analyses of voxel-based morphometry studies that compared a group with uncomplicated chronic alcohol use to healthy controls found mostly similar areas with reduced gray matter volume (Figure 2).8,9 A weakness acknowledged by one was that they were unable to incorporate duration of abstinence, which varied considerably across studies, into their analytics.8 A meta-analysis of MRI studies that compared groups with chronic alcohol use to healthy controls found that both treatment seeking status and number of days abstinent were significant moderators of white matter volume.55 Non-treatment-seeking groups did not differ significantly from healthy controls and the differences in volume between groups with chronic alcohol use and healthy controls decreased as days abstinent increased. More recent studies in individuals with chronic alcohol use entering treatment have also reported that gray matter metrics (total and regional volumes; cortical thickness) were initially smaller (first week of abstinence; first day of abstinence) compared with healthy controls, but white matter volumes were not.12,13 One group reported significant recovery during the first two weeks of abstinence in both gray matter volume and cortical thickness (Figure 3).13,15 The other reported that volumetric increases with abstinence (1 and 7.5 months) were seen in both tissue compartments, with gray matter increasing earlier than white matter.12 Smoking was associated with less recovery in total and frontal gray matter volumes.12 Diffusion tensor imaging has been used to assess white matter integrity in groups with chronic alcohol use compared with healthy controls. Studies performed in early abstinence have found widespread areas of impaired integrity (lower fractional anisotropy) compared with controls (Figure 2).10,11,14 Longitudinal studies indicate that prolonged abstinence is associated with partial normalization of white matter integrity.11,14 Studies suggest that recovery may also occur without total abstinence, which supports reduction efforts for treating alcohol problems when complete abstinence may not be realistic.10,56
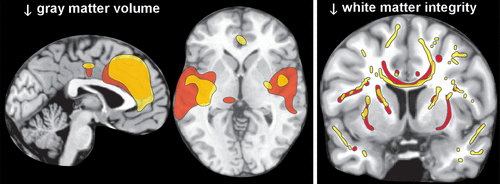
FIGURE 2. Cross sectional magnetic resonance imaging (MRI) studies comparing individuals with uncomplicated chronic alcohol use to healthy controls have reported more widespread differences in gray and white matter than have been found in autopsy studies.6,7 Multiple studies have utilized voxel-based morphometry to identify areas of reduced gray matter. Representative findings from two recent meta-analyses are color-coded (red, yellow) onto axial and sagittal MRIs.8,9 Diffusion tensor imaging has been used to identify areas of reduced white matter integrity, as indicated by lower fractional anisotropy. Representative findings from two recent studies are color-coded (red, yellow) on to a coronal MRI.10,11
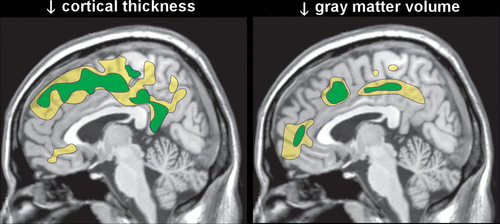
COVER AND FIGURE 3. Longitudinal MRI studies in groups with uncomplicated chronic alcohol use entering treatment have reported improvements in gray matter (total and regional volumes; cortical thickness) and white matter (total and regional volumes; integrity) over varying durations of abstinence.11–14 A study that compared a group with chronic alcohol use to healthy controls on the first and fourteenth days of abstinence reported that areas of difference were greatly reduced by time two, indicating significant recovery in both gray matter volume and cortical thickness.13,15 Representative findings from day one (yellow) and day fourteen (green) for cortical thickness and gray matter volume are color-coded on to sagittal axial (cover) MRIs.
The evidence that alterations in the brain associated with chronic alcohol use can improve measurably in as little as two weeks of abstinence indicates that the brain has a robust ability to repair and/or restructure.6,7 Multiple mechanisms have been proposed for reduced tissue volumes in both gray and white matter including inflammation, oxidative stress, myelin thinning, cell body shrinkage, disruption of intracellular cytoskeletal structure/functioning, dendritic pruning, and suppression of neurogenesis.7,51,57–59 Alcohol-induced liver dysfunction and repeated episodes of subclinical nutritional deficiencies (e.g., thiamine) are also likely to be relevant. Once thought to be important for the reversibility of atrophy due to chronic alcohol use, the rehydration hypothesis has been disproven.6 Although still in the early stages of investigation, processes that may contribute to volumetric recovery include remyelination, regrowth of dendrites, and compensatory increases in neurogenesis.13,58 Longitudinal studies have reported residual differences between chronic alcohol users and healthy controls. Although some of these differences may be indicative of permanent alcohol-related changes, it is likely that greater recovery will be found with increased time abstinent. In addition, there is growing evidence of pre-existing differences that are risk factors for the development of chronic alcohol use.60,61
Problems with sleep have been implicated as a risk factor for both development of problematic alcohol use and relapse during abstinence.17,41,62 Cross-sectional studies in adolescents have reported that sleep-associated factors (e.g., poor sleep habits, evening chronotype, circadian phase delay) correlate with development of many problems including alcohol misuse.17 More recent longitudinal studies of adolescents also support problematic sleep as a risk factor.63–65 In addition, decreases in sleep quality longitudinally predict problematic alcohol use in Navy Veterans following deployment.66 Increase in REM sleep deficit during abstinence has been noted as a possible predictor of vulnerability to relapse.67 Abstinent individuals who have difficulties falling asleep may also be at higher risk for relapse as acute alcohol consumption reverses NREM deficits and decreases sleep latency.16,68 This interpretation is consistent with a recent follow-up study of patients who completed an intensive residential treatment program.69 Both use of hypnotic medications and use of alcohol as a hypnotic at time of admission were associated with relapse by 12 months, but sleep problems at admission or discharge were not. Prioritizing treatment of sleep problems in patients with primary or comorbid chronic alcohol use may reduce the risk of relapse. Early preventative intervention is also pertinent, as sleep problems in adolescents predict later alcohol misuse.17
Both pharmacologic and behavioral options are available to treat sleep problems (e.g., insomnia).41,62,70,71 Evidence regarding the efficacy of using medications to treat sleep problems in this population varies considerably.41,70,71 Trazadone is one of the most commonly prescribed medications for sleep problems in chronic alcohol use.70,71 A recent review of nonpharmacologic approaches to treating sleep problems in patients with alcohol related disorders identified only five relevant studies.62 The study utilizing progressive relaxation training reported improvements in sleep quality. Four studies utilizing cognitive behavioral therapy for insomnia (CBT-I) reported improvements in multiple aspects of sleep and decreases in daytime fatigue. In some instances, CBT-I has been noted as beneficial in helping wean patients off of medication for insomnia.72 However, the efficacy of CBT-I for reducing relapse is unclear.73
In summary, most evidence suggests that chronic alcohol use affects homeostatic sleep drive most prominently, as indicated by significant alterations in NREM and REM sleep, as well as prolonged sleep latency. Although sleep systems are expected to improve with abstinence, previous chronic alcohol use may be a risk factor for relapse in patients presenting with sleep problems. Recent longitudinal studies indicate considerable recovery in gray and white matter volumes with abstinence.
1 : Sleep neurobiology from a clinical perspective. Sleep 2011; 34:845–858Crossref, Medline, Google Scholar
2 : Adenosine, energy metabolism and sleep homeostasis. Sleep Med Rev 2011; 15:123–135Crossref, Medline, Google Scholar
3 : The Neurobiology of Sleep, in Neurobiology of Mental Illness, 4th ed. Edited by Charney DS, Buxbaum JD, Sklar P, . New York, Oxford University Press, 2013, pp 1127–1143Google Scholar
4 : The neurobiology of sleep and wakefulness. Psychiatr Clin North Am 2015; 38:615–644Crossref, Medline, Google Scholar
5 : Sleep-wake regulation and its impact on working memory performance: the role of adenosine. Biology (Basel) 2016; 5:1–25Google Scholar
6 : The neuropathology of alcohol-related brain damage. Alcohol 2009; 44:136–140Crossref, Google Scholar
7 : Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol 2011; 7:284–294Crossref, Medline, Google Scholar
8 : Cortical and subcortical gray matter shrinkage in alcohol-use disorders: a voxel-based meta-analysis. Neurosci Biobehav Rev 2016; 66:92–103Crossref, Medline, Google Scholar
9 : Regional gray matter deficits in alcohol dependence: A meta-analysis of voxel-based morphometry studies. Drug Alcohol Depend 2015; 153:22–28Crossref, Medline, Google Scholar
10 : Relationship between brain volumetric changes and interim drinking at six months in alcohol-dependent patients. Alcohol Clin Exp Res 2014; 38:739–748Crossref, Medline, Google Scholar
11 : White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry 2014; 1:202–212Crossref, Medline, Google Scholar
12 : Serial longitudinal magnetic resonance imaging data indicate non-linear regional gray matter volume recovery in abstinent alcohol-dependent individuals. Addict Biol 2015; 20:956–967Crossref, Medline, Google Scholar
13 : Longitudinal mapping of gyral and sulcal patterns of cortical thickness and brain volume regain during early alcohol abstinence. Eur Addict Res 2016; 22:80–89Crossref, Medline, Google Scholar
14 : Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res 2012; 36:1922–1931Crossref, Medline, Google Scholar
15 : Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcohol Clin Exp Res 2013; 37:67–74Crossref, Medline, Google Scholar
16 : Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: a comprehensive review. Addict Sci Clin Pract 2016; 11:9Crossref, Medline, Google Scholar
17 : Alcohol and the sleeping brain. Handb Clin Neurol 2014; 125:415–431Crossref, Medline, Google Scholar
18 : Association of sleep disturbance with chronicity and remission of alcohol dependence: data from a population-based prospective study. Alcohol Clin Exp Res 2004; 28:1533–1540Crossref, Medline, Google Scholar
19 : Mechanisms underlying sleep-wake disturbances in alcoholism: focus on the cholinergic pedunculopontine tegmentum. Behav Brain Res 2014; 274:291–301Crossref, Medline, Google Scholar
20 : Alcohol disrupts sleep homeostasis. Alcohol 2015; 49:299–310Crossref, Medline, Google Scholar
21
22 : Functional neuroanatomy of sleep and circadian rhythms. Brain Res Brain Res Rev 2009; 61:281–306Crossref, Google Scholar
23 : Neurobiology of circadian rhythm regulation. Sleep Med Clin 2015; 10:403–412Crossref, Medline, Google Scholar
24 : Functional neuroanatomy of sleep and sleep deprivation. J Neuropsychiatry Clin Neurosci 2006; 18:1–5Link, Google Scholar
25 : Circadian rhythms, sleep, and substance abuse. Sleep Med Rev 2012; 16:67–81Crossref, Medline, Google Scholar
26 : Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol Regul Integr Comp Physiol 2009; 297:R729–R737Crossref, Medline, Google Scholar
27 : Circadian clock and stress interactions in the molecular biology of psychiatric disorders. Curr Psychiatry Rep 2014; 16:483–494Crossref, Medline, Google Scholar
28 : Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. J Neurosci 2012; 32:4417–4425Crossref, Medline, Google Scholar
29 : Adenosine and the homeostatic control of sleep: effects of A1 receptor blockade in the perifornical lateral hypothalamus on sleep-wakefulness. Neuroscience 2008; 153:875–880Crossref, Medline, Google Scholar
30 : A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci 2003; 23:4278–4287Crossref, Medline, Google Scholar
31 : Sleep homeostasis and models of sleep regulation. J Biol Rhythms 1999; 14:557–568Medline, Google Scholar
32 : Sleep homeostasis in alcohol-dependent, depressed and healthy control men. Eur Arch Psychiatry Clin Neurosci 2011; 261:559–566Crossref, Medline, Google Scholar
33 : NREM sleep stage transitions control ultradian REM sleep rhythm. Sleep 2011; 34:1423–1432Crossref, Medline, Google Scholar
34 : Implication of the purinergic system in alcohol use disorders. Alcohol Clin Exp Res 2011; 35:584–594Crossref, Medline, Google Scholar
35 : Adenosine and glutamate signaling in neuron-glial interactions: implications in alcoholism and sleep disorders. Alcohol Clin Exp Res 2012; 36:1117–1125Crossref, Medline, Google Scholar
36 : Role of wake-promoting basal forebrain and adenosinergic mechanisms in sleep-promoting effects of ethanol. Alcohol Clin Exp Res 2010; 34:997–1005Crossref, Medline, Google Scholar
37 : Brain pathways to recovery from alcohol dependence. Alcohol 2015; 49:435–452Crossref, Medline, Google Scholar
38 : Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J Neurochem 2010; 115:782–794Crossref, Medline, Google Scholar
39 : Chronic alcohol consumption in rats leads to desynchrony in diurnal rhythms and molecular clocks. Alcohol Clin Exp Res 2016; 40:291–300Crossref, Medline, Google Scholar
40 : Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res 2013; 37:539–549Crossref, Medline, Google Scholar
41 : Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res 2016; 40:2271–2282Crossref, Medline, Google Scholar
42 : Sleep in psychiatric disorders: where are we now? Can J Psychiatry 2010; 55:403–412Crossref, Medline, Google Scholar
43 : Factors that predispose, prime and precipitate NREM parasomnias in adults: clinical and forensic implications. Sleep Med Rev 2007; 11:5–30, discussion 31–33Crossref, Medline, Google Scholar
44 : The within-person association between alcohol use and sleep duration and quality in situ: an experience sampling study. Addict Behav 2016; 61:68–73Crossref, Medline, Google Scholar
45 : Disturbed sleep and its relationship to alcohol use. Subst Abus 2005; 26:1–13Crossref, Medline, Google Scholar
46 : Dim light melatonin onset in alcohol-dependent men and women compared with healthy controls. Chronobiol Int 2012; 29:35–42Crossref, Medline, Google Scholar
47 : Sleep, sleepiness, and alcohol use. Alcohol Res Health 2001; 25:101–109Medline, Google Scholar
48 : Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry 2001; 158:399–404Crossref, Medline, Google Scholar
49 : Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry 2003; 54:1437–1443Crossref, Medline, Google Scholar
50 : Circadian rhythm of REM sleep of chronic alcoholics during alcohol withdrawal. Drug Alcohol Depend 1986; 18:77–85Crossref, Medline, Google Scholar
51 : Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol 2009; 44:115–127Crossref, Google Scholar
52 : The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, Ill, American Academy of Sleep Medicine, 2007Google Scholar
53 : Inversion of melatonin circadian rhythm in chronic alcoholic patients during withdrawal: preliminary study on seven patients. Alcohol 2009; 44:42–45Crossref, Google Scholar
54 : Melatonin and cortisol circadian secretion during ethanol withdrawal in chronic alcoholics. Chronobiologia 1994; 21:109–112Medline, Google Scholar
55 : White matter volume in alcohol use disorders: a meta-analysis. Addict Biol 2013; 18:581–592Crossref, Medline, Google Scholar
56 . Regional brain volume changes in alcohol-dependent individuals during early abstinence: associations with relapse following treatment. Addict Biol (Epub ahead of print, June 22, 2016)Google Scholar
57 : Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol 2008; 210:349–358Crossref, Medline, Google Scholar
58 : Cognitive decline and recovery in alcohol abuse. J Mol Neurosci 2016; 60:383–389Crossref, Medline, Google Scholar
59 : Alcohol-related brain damage in humans. PLoS One 2014; 9:e93586Crossref, Medline, Google Scholar
60 : Adolescent drinking and brain morphometry: a co-twin control analysis. Dev Cogn Neurosci 2015; 16:130–138Crossref, Medline, Google Scholar
61 : Adolescent cortical thickness pre- and post marijuana and alcohol initiation. Neurotoxicol Teratol 2016; 57:20–29Crossref, Medline, Google Scholar
62 : Sleep disturbances in individuals with alcohol-related disorders: a review of cognitive-behavioral therapy for insomnia (CBT-I) and associated non-pharmacological therapies. Subst Abuse 2014; 8:55–62Medline, Google Scholar
63 : Restless sleep and variable sleep timing during late childhood accelerate the onset of alcohol and other drug involvement. J Stud Alcohol Drugs 2016; 77:649–655Crossref, Medline, Google Scholar
64 : A longitudinal study of insomnia and other sleep complaints in adolescents with and without alcohol use disorders. Alcohol Clin Exp Res 2014; 38:2225–2233Crossref, Medline, Google Scholar
65 : The hazards of bad sleep-sleep duration and quality as predictors of adolescent alcohol and cannabis use. Drug Alcohol Depend 2016; 168:335–339Crossref, Medline, Google Scholar
66 : Work stressors, sleep quality, and alcohol-related problems across deployment: A parallel process latent growth modeling approach among Navy members. Stress Health (Epub ahead of print, Oct 10, 2016)Google Scholar
67 : Sleep and the cholinergic rapid eye movement sleep induction test in patients with primary alcohol dependence. Biol Psychiatry 2001; 50:383–390Crossref, Medline, Google Scholar
68 : Treatment options for sleep disturbances during alcohol recovery. J Addict Dis 2007; 26:41–54Crossref, Medline, Google Scholar
69 : The association between sleep disturbances and alcohol relapse: A 12-month observational cohort study. Am J Addict 2015; 24:362–367Crossref, Medline, Google Scholar
70 : Assessing and treating insomnia related to alcohol use disorders. Curr Addict Rep 2016; 3:98–108Crossref, Google Scholar
71 : Pharmacological treatment of insomnia in alcohol recovery: a systematic review. Alcohol 2011; 46:578–585Crossref, Google Scholar
72 : Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction 2004; 99:1121–1132Crossref, Medline, Google Scholar
73 : Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther 2011; 49:227–233Crossref, Medline, Google Scholar



