The Relationship Between Prior Concussions and Depression Is Modified by Somatic Symptomatology in Retired NFL Athletes
Abstract
A positive relationship between sport-related concussion (SRC) history and depressive symptoms in retired National Football League (NFL) athletes has been observed, with self-rated physical functioning identified as a confounding factor. The authors examined the influence of somatic symptom endorsement on the relationship between SRC history and self-reported depressive symptom severity in retired NFL athletes. Forty-three former NFL athletes completed self-report inventories of depression (with the Beck Depression Inventory II) and somatic symptoms (with the adjusted Patient Health Questionnaire-15). A moderation analysis examined the influence of somatic symptoms on the relationship between SRC history and depressive symptom severity. SRC history and somatic symptoms accounted for a significant amount of depressive symptomology. SRC history was not significantly associated with depressive symptom severity at low levels of somatic symptoms but was significant at the mean and high levels. The effect of somatic symptoms on depressive symptoms was nearly twice that of SRC history. The relationship between SRC and depression is complex, and treatment of depression in retired athletes should address comorbid somatic symptoms.
Previous studies have suggested that a relationship exists between sport-related concussion (SRC) and adverse neuropsychological outcomes, including depression, in later life.1–3 Understanding the potential long-term effects of SRC on depression risk could have profound public health implications, given that an estimated 1.6 to 3.8 million SRCs occur yearly.4–6 However, the empirical relationship between mild traumatic brain injury (mTBI) and depression is complex due to overlapping, nonspecific symptoms, comorbid pathology, and substance or medication use.7,8
According to DSM-5, the clinical diagnosis of depression is based on the assessment of a diverse set of clinical symptoms and requires the presence of at least five out of nine criteria-based symptoms during a 2-week period that cause clinically significant distress or impaired functioning.9 Symptoms involve multiple domains, including affective (depressed mood, feelings of worthlessness, and recurrent thoughts of death), cognitive (diminished ability to think or concentrate), and somatic changes (sleep disturbance, fatigue, psychomotor retardation, decreased libido, and significant weight loss).10
National Football League (NFL) athletes have been studied extensively to elucidate the relationship between SRC and depression, given their prolonged exposure to repetitive head contact or SRC.11 In 2007, Guskiewicz and colleagues2 first observed a positive relationship between number of self-reported SRCs and self-reported diagnosis of depression among 2,522 NFL retirees. Compared with athletes with no history of SRC, those with one to two self-reported SRCs were 1.5 times more likely to report a diagnosis of depression, and those with three or more SRCs were 3 times more likely to do so. Five years later, the same group published a second analysis of 1,044 retired NFL athletes and found that the relationship between self-reported SRCs and risk of self-reported depression was dose dependent across a 9-year interval.1
More recent studies have used psychometric instruments to assess for depression, rather than self-report, and findings have been mixed.3,12 In a study of 26 retired NFL athletes, Strain et al.12 did not observe a significant difference in lifetime SRCs between those who fell above and below a standardized depression cut-off score using the Beck Depression Inventory II (BDI-II). However, a study of 30 retired NFL athletes by Didehbani and colleagues3 did demonstrate that self-reported SRCs correlated positively with BDI-II scores as a continuous variable when no cut-off score or diagnosis of depression was used.3 Furthermore, the mean BDI-II depression score for retired NFL athletes was 8.80, falling in the minimal range and raising questions regarding clinical meaningfulness. Didehbani and colleagues3 also compared NFL retirees to a well-matched control group; while significant differences were observed on all three BDI-II subscores (affective, cognitive, and somatic), the largest difference was observed with somatic symptoms (4.38 versus 1.79, respectively).3
Given the rigorous physical demands of professional football, it is not uncommon for NFL retirees to endorse multiple somatic symptoms, especially pain,13 that could affect their concentration, appetite, energy, and sleeping habits.14–16 Additionally, the negative correlation between greater SRC history (≥3) and self-reported physical health has been previously observed in current and former collegiate athletes, suggesting a potential relationship between SRC history and the degree of somatic symptoms experienced by former athletes.17 In the two earliest studies of depression and NFL retirees,1,2 Short Form Physical Component Score (SF-36 PCS) was significantly correlated with depression, where more physical disability related to more severe depression. Although physical symptoms were assessed in these studies, the true impact of somatic symptoms as they relate to depression and SRC history has not been studied. Thus, further investigation into the relationships between SRC history, depressive symptom severity, and somatic symptom endorsement is warranted.
In the current study, we sought to determine whether somatic symptom endorsement moderates the relationship between SRC history and depressive symptom severity in retired NFL athletes. Based on the higher rates of somatic symptoms endorsed by NFL athletes,3,13 as well as independent paired relationships between SRC history, depressive symptom severity, and somatic or physical symptoms,1,2,17 we hypothesized that the relationship between SRC history and depression symptom severity would be stronger at higher levels of somatic symptom endorsement and weaker at lower levels of somatic symptom endorsement.
Methods
Participants
Participants were originally recruited for a study assessing neurologic function in former NFL athletes.18 Recruitment methods, exclusion criteria, and data-gathering procedures have been described in depth elsewhere.18 Below, we provide abbreviated methodological details that are pertinent to the current study. The study coordinator obtained consent for participants who met the inclusion or exclusion criteria. Records were deidentified, coded numerically, and imported into an Excel spreadsheet (Microsoft). Institutional review board-approval for this study was obtained (institutional review board number, 0706004996).
Recruitment
With the help of the NFL Players Association (NFLPA), recruitment letters explaining the purpose and nature of the study were mailed to more than 5,000 retired athletes. Recipients interested in participating were asked to call the study coordinator at a confidential telephone number. Once enrolled, some participants voluntarily contacted former teammates to solicit study participation as well. Two athletes (4.4%) in the final cohort independently contacted the study coordinator after hearing about the study from former teammates. The study coordinator also called athletes at random from NFLPA records in order to invite their participation.
Inclusionary and Exclusionary Criteria
The inclusion criteria were former NFL athletes interested in participation, age 60 years or less, who were able to tolerate MRI. The exclusionary criteria were a history of brain surgery; brain tumors; cerebrovascular accidents, such as stroke; multiple sclerosis; seizures that began before entering the NFL (except febrile seizures); HIV or AIDS; nonathletic trauma that resulted in significant head injuries; complicated concussions or mTBI that involved loss of consciousness for more than 1 minute or hospitalization that took place after NFL career; open heart surgery, organ transplant surgery, or carotid artery surgery; cancer-related treatment (e.g., chemotherapy or radiation therapy) that contained the potential for adverse effects on the brain or spinal cord functioning; renal failure requiring dialysis, liver failure, cirrhosis, or request for liver transplant; substance-use-related league suspension, arrest for driving under the influence, or treatment in a rehabilitation facility for drug or alcohol abuse, indicating the presence of significant alcohol or drug abuse in the past or present; current daily use of an illegal drug; and suspected current alcohol dependence disorder, as indicted by daily intake of more than four beers or more than two liquor drinks per day during the past 5 years.
Demographic Characteristics and Exposure
A thorough medical, social, and family history was obtained from each participant. After participants were read the definition of a concussion used by the NFL’s mTBI Committee,19 a detailed head injury and concussion history was obtained from every participant, including recall of the number of concussions sustained at all levels of play. SRC history was examined as a continuous variable.
Measures
A comprehensive battery of self-report inventories was administered and scored by a board-certified neuropsychologist (PhD), who used standardized scoring instructions. The focus of the current study was the BDI-II15 and the Patient Health Questionnaire (PHQ) results.20,21 The BDI-II is a frequently used self-report questionnaire for the assessment of clinical depression.20,21 Participants rated the severity of the 21 depressive symptoms on a four-point scale (0–3). Greater scores indicated higher symptomology. Total scores could range from 0 to 63 and were examined as a continuous variable. Ranges for interpretation of BDI-II total scores include 0–13 (minimal), 14–19 (mild depression), 20–28 (moderate depression), and 29–63 (severe depression).22
Somatic symptoms were measured using the Patient Health Questionnaire-15 (PHQ-15)23 and scored using standardized scoring instructions. As part of the full PHQ, originally designed to screen for multiple common mental health disorders in primary care patients,24 the PHQ-1525 is a subscale of a larger instrument that measures commonly experienced somatization or physical complaints. Three items were removed from the original PHQ-15 for the purposes of this study. One was a question about menstrual cramps. Two additional items were removed due to direct overlap with similar BDI-II questions: 1) feeling tired or having low energy, and 2) trouble sleeping. The final 12 items were scored from 0 to 2, with 0 indicating responses of “not bothered” and 2 corresponding with “bothered a lot” by the particular somatic symptom. Total somatic symptom endorsement scores on the adjusted PHQ-15 could range from 0 to 24 and were examined as a continuous variable.
Statistical Analysis
Outliers or extreme values in the data were detected using the outlier labeling rule.26 A moderation analysis was used to investigate the main effects of somatic-related symptoms and SRC history on depressive symptom severity, as well as an interaction effect between SRC history and somatic symptoms.27,28 Mean centering of the predictor variables was performed in order to reduce likely multicollinearity.28,29 As part of the moderation analysis, follow-up simple slopes analysis (effect of SRC history on depression at 1 standard deviation below, 1 standard deviation above, and at the mean of somatic symptoms) was performed in order to further investigate the nature of the interaction observed.30 Additionally, the Johnson-Neyman technique was performed to identify the exact threshold at which somatic symptom endorsement influences SRC history and depression scores.31
Results
Demographic and Symptom Characteristics
After detecting and removing outliers from the 45 retired NFL athletes who met inclusion or exclusion criteria, 43 (96%) retired athletes were included in the final moderation analysis. Outliers of SRC history (25; N=1) and depression severity (35; N=1) were removed in order to reduce skewness associated with normality assumptions in the data. The retired NFL players in this cohort averaged 8.7 (SD=6.63) prior SRCs. Complete demographic characteristics of the final sample are provided in Table 1. The average total depression score on the BDI-II questionnaire was 9.26 (SD=8.95). The frequency of individual depressive symptom endorsement is provided in Table 2. Of the total sample with BDI-II scores, 29 subjects fell within the minimal depression range (0–13).22 Other depression score classifications across the remaining subjects included nine in the mild,14–19 three in the moderate,20–28 and two in the severe ranges.29 Somatic symptom endorsement was robust across items, averaging a total somatic symptom score of 5.35 (SD=3.58) (Table 3). Somatic symptom endorsement was positively correlated with the total number of SRCs, r=0.36, p=0.02.
| Characteristic | Mean | SD |
|---|---|---|
| Age (years) | 46.35 | 9.16 |
| Height (inches) | 74.98 | 1.77 |
| Weight (lbs) | 255.56 | 46.74 |
| Body mass index (kg/m2) | 31.48 | 4.88 |
| Education | 15.53 | 1.08 |
| Previous sport-related concussions | 8.7 | 6.63 |
| Football experience | ||
| Pre-high school | 2.48 | 2.28 |
| High school | 3.51 | 0.94 |
| College | 4.19 | 0.45 |
| NFL | 6.72 | 3.16 |
| N | % | |
| NFL position | ||
| Quarterback | 0 | 0.0 |
| Running back/fullback | 1 | 2.3 |
| Wide receiver | 2 | 4.7 |
| Tight end | 1 | 2.3 |
| Offensive lineman | 8 | 18.6 |
| Defensive lineman | 8 | 18.6 |
| Safety/cornerback | 8 | 18.6 |
| Linebacker | 13 | 30.2 |
| Disability | ||
| Learning disability | 5 | 11.6 |
TABLE 1. Demographic Characteristics of the Study Sample
| Depressive Symptoms | Endorsed | |
|---|---|---|
| Na | (%)b | |
| Sadness | 14 | 32.6 |
| Pessimism | 14 | 32.6 |
| Past failure | 10 | 23.3 |
| Loss of pleasure | 18 | 41.9 |
| Guilty feelings | 13 | 30.3 |
| Punishment feelings | 7 | 16.3 |
| Self-dislike | 7 | 16.3 |
| Self-criticalness | 21 | 48.8 |
| Suicidal thoughts | 6 | 14.0 |
| Crying | 10 | 23.3 |
| Agitation | 18 | 41.9 |
| Loss of interest | 15 | 34.9 |
| Indecisiveness | 15 | 34.9 |
| Worthlessness | 6 | 14.0 |
| Loss of energy | 27 | 62.8 |
| Changes in sleep pattern | 25 | 58.2 |
| Irritability | 18 | 41.9 |
| Changes in appetite | 14 | 32.6 |
| Concentration difficulty | 19 | 44.2 |
| Tiredness or fatigue | 21 | 48.8 |
| Loss of interest in sex | 18 | 41.9 |
| Total scorec | 9.26d | 8.95e |
TABLE 2. Depression Symptom Endorsement
| Somatic/Physical Symptoms | Endorsed | |
|---|---|---|
| Na | %b | |
| Stomach pain | 9 | 20.9 |
| Arm, leg, or joint pain | 39 | 90.7 |
| Back pain | 33 | 76.8 |
| Pain or problems during sex | 5 | 11.7 |
| Headaches | 21 | 48.9 |
| Chest pain | 5 | 11.7 |
| Dizziness | 14 | 32.5 |
| Fainting spells | 1 | 2.3 |
| Feeling your heart pound or race | 15 | 34.9 |
| Shortness of breath | 10 | 23.3 |
| Constipation, loose bowels | 9 | 20.9 |
| Nausea, gas, or indigestion | 16 | 37.2 |
| Total scorec | 5.35d | 3.58e |
TABLE 3. Somatic Symptom Endorsement
Moderation Analysis
To test the hypothesis that the relationship between SRC history and depression symptom severity is influenced by somatic symptoms, a hierarchal multiple regression analysis was performed. In the first step, SRC history and somatic symptom scores were included, and they accounted for a significant amount of depressive symptomology in retired NFL athletes, R2=0.47, F=17.70, df=2, 40, p<0.001. This indicates a positive association between somatic symptoms and depression scores, as well as number of concussions and depressive symptoms (i.e., greater somatic symptom endorsement or number of concussions increases likelihood of higher depression scores). Standardized beta coefficients of the two variables in the first entry indicated that somatic symptoms, β=0.53, p<0.001, have nearly twice the effect on depressive symptoms above SRC history, β=0.28, p=0.03. For an increase of one standard deviation in somatic symptom score (3.58), predicted depression symptoms increase by 0.53 of a standard deviation (4.74), whereas an increase of one standard deviation of prior SRC (6.63) increased predicted depressive symptom severity by 0.28 of a standard deviation (2.51).
When the interaction term between SRC history and somatic symptom endorsement was entered into the second step of the regression, it explained a significant increase of variance in depressive symptom severity, ΔR2=0.05, F=5.05, df=1, 39, p=0.03. The overall moderation analysis model containing independent predictors of SRC history, somatic symptom endorsement, and their interaction effect on depression symptom severity was significant, R2=0.52, F=15.71, df=3, 39, p<0.001. Main effects of SRC history, B=0.33, p=0.03, and somatic symptom endorsement, B=1.25, p=0.01, remained significant with the interaction effect included.
Slopes Analysis
A simple slopes analysis revealed that SRC history was not significantly associated with depressive symptom severity for somatic symptom endorsement one standard deviation below the mean, B=0.02, p=0.90 (Table 4). At the mean level of somatic symptom endorsement, SRC history was significantly associated with depressive symptom severity, B=0.33, p=0.03. At levels of one standard deviation above the mean of somatic symptom endorsement, the relationship between SRC history and depressive symptom severity was the strongest, B=0.64, p=0.01 (Figure 1). The Johnson-Neyman technique revealed that the relationship between SRC history and severity of depressive symptoms was significant when total somatic symptom endorsement was 5 or greater on the adjusted PHQ-15, with 51% of the sample falling above this threshold (Table 5).
| Predictor | Coefficient (Β) | SE | t | p | 95% CI |
|---|---|---|---|---|---|
| SRC history | 0.33 | 0.15 | 2.21 | 0.03 | 0.03, 0.64 |
| Somatic symptom endorsement | 1.25 | 0.48 | 2.62 | 0.01 | 0.01, 0.21 |
| SRC history by somatic symptomsb | 0.09 | 0.04 | 2.25 | 0.03 | 0.01, 0.17 |
TABLE 4. Moderation Effects of Somatic Symptom Endorsement on Sport-Related Concussion (SRC) History and Depression Symptom Endorsementa
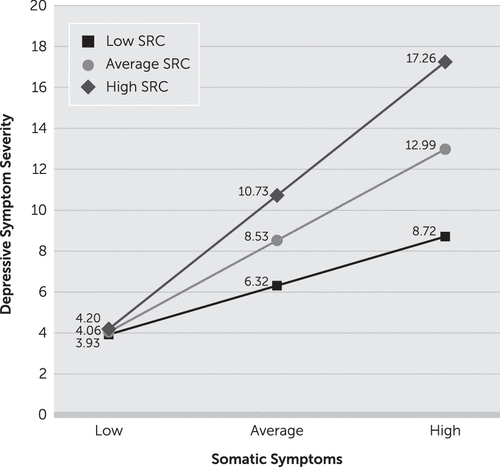
FIGURE 1. Conditional Relationship of Sport-Related Concussion (SRC) History and Depressive Symptom Severity Based on the Degree of Somatic Symptom Reportinga
a Levels of SRC history and somatic symptom reporting are defined as follows: low=1 standard deviation below the sample means; average=mean; high=1 standard deviation above the mean.
| Somatic Symptoms | Coefficient (Β) | SE | t | p | 95% CI |
|---|---|---|---|---|---|
| One standard deviation below the mean (–3.58) | 0.02 | 0.16 | 0.13 | 0.9 | –0.3, 0.34 |
| Mean (0.0) | 0.33 | 0.15 | 2.21 | 0.03 | 0.03, 0.64 |
| One standard deviation above the mean (3.58) | 0.64 | 0.24 | 2.66 | 0.01 | –0.16, 1.13 |
TABLE 5. Conditional Effects of Sport-Related Concussion (SRC) History on Depressive Symptom Severitya
Discussion
Findings from the current study demonstrated that the relationship between SRC history and self-reported depressive symptoms in retired NFL athletes is significantly moderated by somatic symptom endorsement. No association between SRC history and self-reported depressive symptoms on the BDI-II was seen at low levels of somatic symptom endorsement. However, at moderate and high levels of somatic symptom endorsement, a significant relationship between SRC history and self-reported depressive symptoms on the BDI-II was observed.
The current results provide further insight into the previously demonstrated association between SRC history and self-reported depression in retired NFL athletes.1–3 In these previous studies, self-reported physical disability was accounted for through the Short-Form 12 Health Survey Physical Component Summary (SF-12 PCS) score, yet specific somatic symptoms were not tested. While there is significant overlap between somatic symptoms and physical disability status,32 the concept of perceived disability has been more strongly associated with depressive symptoms than actual physical disability.33 Given this difference of perceived versus actual disability, the exclusive study of somatic symptomatology serves as a more specific variable to assess in the relationship between SRC history and depression. Furthermore, somatic symptom endorsement is perhaps a more effective moderator in this complex relationship.
Compared with the two earlier studies that controlled for SF-12 PCS scores, the later study by Didehbani et al.3 did not control for physical symptoms. The authors concluded that depression symptom severity was attributable to SRC history only, despite finding that concussion history was only associated with cognitive factors on the BDI-II. Notably, the largest differences between the NFL retirees and age-matched controls were in the areas of concentration, appetite, loss of energy, sleep, and loss of interest in sex—all somatic complaints. Interestingly, the authors noted that the NFL retirees “did not realize their somatic complaints may reflect symptoms associated with depression” and “as a result, athletes did not acknowledge or endorse feeling ‘depressed’ despite the high endorsement of somatic symptoms on the BDI-II.” As suggested by the current study, without properly controlling for somatic symptoms, a potentially spurious correlation between concussion history and depression may be found, especially in cases where the depression diagnosis is heavily reliant on and influenced by somatic symptom endorsement.
The importance of somatic symptoms may be partially explained by the concept of compounded injuries of both SRC and other musculoskeletal ailments. Retired NFL athletes who endorsed a history of concussion have been shown to also have a greater number of musculoskeletal injuries, which may be due to style of play or altered biomechanics following SRC.34–38 Furthermore, prior musculoskeletal injuries may cause these athletes to become more sedentary later in life, which has been shown in a sample of 1,165 retired athletes to be a risk factor for depression.39 Additionally, a sedentary lifestyle resulting from physical limitations may lead to chronic medical conditions such as diabetes40 or heart disease,41 which can impact depressive symptoms.42 Lastly, increased depressive symptoms may occur if compounded injuries resulted in forced retirement, as voluntary retirement has been associated with increased life satisfaction.43
An alternate explanation is that somatization was a downstream result of the inciting depression. A strong relationship between somatization and depression is a widely accepted notion.44 In a sample of 2,091 primary care patients, over 50% presented with comorbidities of depression, somatization, and anxiety.45 In the general population, individuals diagnosed with depression or somatoform disorder are significantly more likely to visit their general practitioners for health-related care.46 Given the cross-sectional nature of our study, directionality and causation cannot be established. Thus, it cannot be determined whether depression precipitated somatic symptoms or vice versa.
Clinical Implications
These results suggest that medical professionals should be especially attentive to a retired athlete’s comorbid medical history and physically related conditions. If a retired athlete reports an extensive history of SRC and somatic or depressive symptomatology, referral to a sports psychiatrist or clinical neuropsychologist is recommended for further assessment of the primary etiology of these symptoms and to differentiate depressive symptoms as purely somatic, psychiatric, or mixed. Additionally, integrated treatment among providers is necessary for both depression and somatic conditions. For example, pharmacological treatment and counseling management of both depression and pain require communication between practitioners.47
Future Directions
If somatic symptoms can be mitigated, it is possible that the risk and/or severity of depression may be decreased. In this light, how does one protect against somatic symptom development? A career of playing professional football is sure to have negative musculoskeletal effects that may lead to somatic symptoms. Research into inflammatory mechanisms underlying the complex relationship between head trauma,48 musculoskeletal injuries,49 somatic symptoms,50 depression,50,51 and neurodegenerative disorders52,53 is a herculean endeavor, but addressing these physical complaints is a necessity. Our results allow us to conclude that there are moderating factors that can affect the strength and nature of the relationship between SRC and depression. As the scientific community and public interpret the new wave of studies regarding SRC and mTBI in youth, high school, collegiate, and professional athletes, one must aim to account for all variables and not simply interpret a nuanced relationship as the result of SRC alone.
When assessing neurologic function of retired professional athletes, future studies should integrate full medical and psychiatric histories to control for potential confounding variables, in order to gain further insight into the exact mechanism driving this moderated relationship. Moreover, inventories that differentiate somatic symptoms as being psychiatric or medical in origin should also be obtained.54 Screening instruments alone may not discern nuances in the complex relationship between depression and SRC history. Given increased effectiveness, as compared with screening instruments,55 as well as the nuanced relationship of variables in the current study, clinical neuropsychiatric diagnostic interviews should be employed in order to effectively identify a diagnosis of depression in former athletes. Finally, future efforts should bridge somatic symptomatology with functional measures, such as quality of life.
Limitations
The current study is not without limitations. First, the lack of a control group prevents concluding whether the influence of somatic symptom endorsement on the relationship between SRC history and self-reported depression is applicable to all athletes or is specific to the current sample of retired NFL athletes. Additionally, the lower response rate (45 of 5,000 letters sent to former players) warrants caution regarding the external validity and generalizability of the study. As highlighted above, the current analysis did not control for physical conditions or diagnoses. Thus, because the true etiology of somatic symptoms was unknown, it is difficult to know whether the moderating effect of somatic symptoms is due to secondary neurological or psychiatric disorders, other physical conditions, or emotional distress. Additionally, the mean depression score of the current sample, 8.7 (SD=6.63), was not clinically significant, as it fell within the minimal symptom range according to standard BDI-II criteria.22 As such, average self-reported depressive symptom scores in the current study should not be interpreted as reflecting clinical depression. The average depression score in the current study and sample is in alignment with self-reported depressive symptom endorsement (8.74 [SD=9.7]) in a nonpsychiatric sample of adults presenting to their primary care physician.56 As a related limitation of the study, diagnoses of depression were not confirmed via structured clinical interviews by psychiatric clinicians. Furthermore, comorbid psychiatric diagnoses and symptoms were also not taken into account. Similarly, all SRC histories were self-reported, which is not always representative of true injury history.1 The self-report nature of SRC has implications for possible confounds related to tendency to overreport SRCs along with depressive and somatic symptoms. More comprehensive measures with symptom validity testing would likely have been useful in detecting this trend.
Conclusions
The current findings demonstrate that the relationship between SRC history and depressive symptom severity may be significantly moderated by somatic symptom endorsement in retired NFL athletes. An association between SRC history and self-reported depressive symptom severity at low levels of somatic symptom endorsement was not observed. The relationship between SRC history and self-reported depressive symptom endorsement increased linearly, with a stronger association observed at higher somatic symptom levels over moderate or average levels. Additionally, the effect of somatic symptoms on depressive symptoms was nearly twice that of SRC history. Future research is required to determine the mechanism by which somatic symptom endorsement influences SRC history and self-reported depressive symptoms.
1 : Nine-year risk of depression diagnosis increases with increasing self-reported concussions in retired professional football players. Am J Sports Med 2012; 40:2206–2212Crossref, Medline, Google Scholar
2 : Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc 2007; 39:903–909Crossref, Medline, Google Scholar
3 : Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol 2013; 28:418–424Crossref, Medline, Google Scholar
4 : The epidemiology of sport-related concussion. Clin Sports Med 2011; 30:1–17, viiCrossref, Medline, Google Scholar
5 : The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006; 21:375–378Crossref, Medline, Google Scholar
6 : Depression as a modifying factor in sport-related concussion: a critical review of the literature. Phys Sportsmed 2016; 44:14–19Crossref, Medline, Google Scholar
7 : Predicting depression following mild traumatic brain injury. Arch Gen Psychiatry 2005; 62:523–528Crossref, Medline, Google Scholar
8 : Psychiatric disorders following traumatic brain injury: their nature and frequency. J Head Trauma Rehabil 2009; 24:324–332Crossref, Medline, Google Scholar
9 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Publishing, 2013Crossref, Google Scholar
10 : A psychometric evaluation of the BDI-II in treatment-seeking substance abusers. J Subst Abuse Treat 2001; 20:197–204Crossref, Medline, Google Scholar
11 : Estimating contact exposure in football using the head impact exposure estimate. J Neurotrauma 2015; 32:1083–1089Crossref, Medline, Google Scholar
12 : Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology 2013; 81:25–32Crossref, Medline, Google Scholar
13 : Whole-person impairment in younger retired NFL players: the orthopaedic toll of a professional football career. Orthop J Sports Med 2014; 2:2325967114534824Crossref, Google Scholar
14 : Is chronic nonmalignant pain associated with decreased appetite in older adults? Preliminary evidence. J Am Geriatr Soc 2004; 52:247–251Crossref, Medline, Google Scholar
15 : Attention and somatic awareness in chronic pain. Pain 1997; 72:209–215Crossref, Medline, Google Scholar
16 : Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain 1998; 14:311–314Crossref, Medline, Google Scholar
17 : Current physical and mental health of former collegiate athletes. Orthop J Sports Med 2014; 2:2325967114544107Crossref, Google Scholar
18 : Is there chronic brain damage in retired NFL players? Neuroradiology, neuropsychology, and neurology examinations of 45 retired players. Sports Health 2014; 6:384–395Crossref, Medline, Google Scholar
19 : Background on the National Football League’s research on concussion in professional football. Neurosurgery 2003; 53:797–798Crossref, Medline, Google Scholar
20 : Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res (Hoboken) 2011; 63(Suppl 11):S454–S466Medline, Google Scholar
21 : Assessment of depression in medical patients: a systematic review of the utility of the Beck Depression Inventory-II. Clinics (São Paulo) 2013; 68:1274–1287Crossref, Medline, Google Scholar
22 : Manual for the Beck Depression Inventory-II. San Antonio, Tex, Psychological Corporation, 1996Google Scholar
23 : Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999; 282:1737–1744Crossref, Medline, Google Scholar
24 : The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010; 32:345–359Crossref, Medline, Google Scholar
25 : The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002; 64:258–266Crossref, Medline, Google Scholar
26 : Performance of some resistant rules for outlier labeling. J Am Stat Assoc 1986; 81:991–999Crossref, Google Scholar
27 : The impact of attention deficit hyperactivity disorder on recovery from mild traumatic brain injury. J Neurosurg Pediatr 2013; 12:97–102Crossref, Medline, Google Scholar
28 : Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 2nd ed. New York, Guilford Press, 2017Google Scholar
29 : Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA, Sage Publications, 1991Google Scholar
30 : Probing three-way interactions in moderated multiple regression: development and application of a slope difference test. J Appl Psychol 2006; 91:917–926Crossref, Medline, Google Scholar
31 : Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods 2009; 41:924–936Crossref, Medline, Google Scholar
32 : Longitudinal relationship between pain and depression in older adults: sex, age and physical disability. Soc Psychiatry Psychiatr Epidemiol 2002; 37:23–30Crossref, Medline, Google Scholar
33 . Physical functioning, perceived disability, and depressive symptoms in adults with arthritis. Arthritis. 2013;2013:525761.Crossref, Medline, Google Scholar
34 : Concussion frequency associates with musculoskeletal injury in retired NFL players. Med Sci Sports Exerc 2015; 47:2366–2372Crossref, Medline, Google Scholar
35 : Lower extremity stiffness changes after concussion in collegiate football players. Med Sci Sports Exerc 2017; 49:167–172Crossref, Medline, Google Scholar
36 : Concussion may increase the risk of subsequent lower extremity musculoskeletal injury in collegiate athletes. Sports Med 2017; 47:1003–1010Crossref, Medline, Google Scholar
37 : Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med 2016; 44:742–747Crossref, Medline, Google Scholar
38 : Lower extremity musculoskeletal injury risk after concussion recovery in high school athletes. J Athl Train 2017; 52:1028–1034Crossref, Medline, Google Scholar
39 : Influence of physical activity on depression and anxiety of former elite athletes. Int J Sports Med 2003; 24:609–619Crossref, Medline, Google Scholar
40 : Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 2003; 289:1785–1791Crossref, Medline, Google Scholar
41 : Changes in physical activity, mortality, and incidence of coronary heart disease in older men. Lancet 1998; 351:1603–1608Crossref, Medline, Google Scholar
42 : Demographic and psychosocial correlates of physical activity in late life. Am J Prev Med 2001; 21:306–312Crossref, Medline, Google Scholar
43 : Amantadine and cognitive flexibility: decision making in Parkinson’s patients with severe pathological gambling and other impulse control disorders. Neuropsychiatr Dis Treat 2014; 10:1093–1101Crossref, Medline, Google Scholar
44 : Somatization and depression. Psychosomatics 1990; 31:13–21Crossref, Medline, Google Scholar
45 : Depression, anxiety and somatization in primary care: syndrome overlap and functional impairment. Gen Hosp Psychiatry 2008; 30:191–199Crossref, Medline, Google Scholar
46 : Depression, anxiety, and somatoform disorders: vague or distinct categories in primary care? Results from a large cross-sectional study. J Psychosom Res 2009; 67:189–197Crossref, Medline, Google Scholar
47 : Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA 2009; 301:2099–2110Crossref, Medline, Google Scholar
48 : Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab 2014; 34:1223–1232Crossref, Medline, Google Scholar
49 : What do we mean by the term “inflammation”? A contemporary basic science update for sports medicine. Br J Sports Med 2004; 38:372–380Crossref, Medline, Google Scholar
50 : Activation of cell-mediated immunity in depression: association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog Neuropsychopharmacol Biol Psychiatry 2012; 36:169–175Crossref, Medline, Google Scholar
51 : Inflammation in depression and the potential for anti-inflammatory treatment. Curr Neuropharmacol 2016; 14:732–742Crossref, Medline, Google Scholar
52 : The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl Neurodegener 2016; 5:7Crossref, Medline, Google Scholar
53 : Chronic exposure to androgenic-anabolic steroids exacerbates axonal injury and microgliosis in the CHIMERA mouse model of repetitive concussion. PLoS One 2016; 11:e0146540Crossref, Medline, Google Scholar
54 : A-MUPS score to differentiate patients with somatic symptom disorder from those with medical disease for complaints of non-acute pain. J Pain Res 2017; 10:1411–1423Crossref, Medline, Google Scholar
55 : Factor structure and diagnostic validity of the Beck Depression Inventory-II with adult clinical inpatients: comparison to a gold-standard diagnostic interview. Psychol Assess 2014; 26:1106–1115Crossref, Medline, Google Scholar
56 : Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol 2001; 20:112–119Crossref, Medline, Google Scholar



