Emotional Reactivity as a Vulnerability for Psychogenic Nonepileptic Seizures? Responses While Reliving Specific Emotions
Abstract
Objective:
Dysfunction in emotional processes is a hypothesized contributor to functional neurological disorders (FNDs), yet few studies have evoked real-time emotion during multimethod assessment incorporating subjective, behavioral, and psychophysiological indicators. This approach may reveal clinical and neurobiological vulnerability to FND and clarify how dysfunctional emotional processes serve as perpetuating factors.
Methods:
Eleven participants with video-EEG-confirmed diagnoses of psychogenic nonepileptic seizures (PNES) were compared with 49 seizure-free trauma control subjects (TCs) with or without clinically elevated posttraumatic stress symptoms (25 clinically elevated [TC-clin], 24 not clinically elevated [TC-nonclin]). Participants recalled and described memories evoking anger, shame, happiness, and neutral feelings.
Results:
Even though PNES patients and TCs reported similar amounts of emotional experience, PNES patients reported more difficulty reliving emotions and were less likely to complete the relived shame task. During and after reliving happiness, PNES and TC-clin groups showed respiratory sinus arrhythmia (RSA) decreases, indicating parasympathetic withdrawal, whereas the TC-nonclin group showed RSA increases.
Conclusions:
Findings from this pilot study are consistent with previous research and clinical observations that emotional engagement may be more effortful for PNES patients. Patterns of RSA change, which may also point to greater effortful engagement, were similar in PNES and TC-clin groups, suggesting that traumatic stress reactions may play a part. At the same time, experience of greater difficulty or avoidance may be even greater among PNES patients. Especially when regulatory resources are already limited, accumulated effort, coupled with self-threatening contexts such as shame, may be particularly problematic for those with PNES and perhaps other FNDs.
Dysfunction in emotional processes is a key hypothesized contributor to functional neurological disorders (FNDs). One FND is psychogenic nonepileptic seizures (PNES), seizure-like symptoms without EEG-based epileptiform activity. Compared with epilepsy patients or healthy controls, individuals with PNES show heightened preconscious attention to threat (anger displays) (1), emotional avoidance (2, 3), higher basal cortisol (1), lower baseline heart-rate variability (1, 4), and higher resting-state connectivity per functional MRI (5, 6). Few studies, however, have elicited and measured psychophysiological, behavioral, and subjective indicators during real-time emotional reactivity. This approach may suggest how dysfunctional emotional processes contribute to PNES. For example, in PNES, interoceptive signals from the autonomic nervous system may escape integration into ordinary emotion processes, leading to somatic sensations or motor outputs that are disconnected from typical emotional episodes (5–11).
We examined emotional reactivity in PNES patients and comparison participants during a relived emotions task (12); participants recalled and described autobiographical events evoking real-time emotional states. In previous research this task has evoked high levels of target emotional experiences (e.g., anger, happiness) and concomitant physiological changes (12, 13). When PNES patients are asked to relive emotions, we can observe the kinds of events they choose, the extent of their emotional engagement or avoidance, and whether confrontation with emotions activates internal responses or perceptions (e.g., rapid heartbeat) that could be proximal PNES precursors or triggers (5, 11). We examined three discrete emotions potentially implicated in PNES: shame and anger, which are important in trauma-related disorders (13–15) and may trigger avoidance mechanisms hypothesized as central in PNES and other FNDs (2, 3, 5, 7); and happiness, given deficits in positive emotion in posttraumatic stress disorder (PTSD) (14) and our previous finding of blunted positive emotional displays in PNES (4). We assessed cardiac interbeat intervals (IBIs) and respiratory sinus arrhythmia (RSA; or high-frequency heart-rate variability) as indicators of internal emotional arousal. Finally, because trauma has accounted for many emotion findings in prior PNES studies (1, 4, 16, 17), we included two groups of trauma controls (TCs) with high and low levels of posttraumatic stress symptoms (PTS), respectively.
We hypothesized that compared with TCs with or without clinically-elevated PTS, individuals with PNES would display greater difficulty engaging in relived emotion tasks (per self-report and willingness to do the tasks), report feeling less of the target emotion, and show greater increases in cardiovascular arousal (shorter IBIs, reflecting faster heart rate, and larger RSA decreases, reflecting less parasympathetic engagement) during and immediately after reliving.
Methods
Recruitment and procedures were approved by the university’s committee for the protection of human subjects; compensation was $75. Participants completed online or mailed questionnaires and then attended one 3-hour laboratory session. Data collection took place between November 2010 and December 2015.
PNES patients (N=11; women, N=9) had video-EEG-confirmed diagnoses determined by board-certified clinical neurophysiologists or epileptologists. None had epileptic seizures, mixed seizure type, or unclear diagnoses. Mean age at PNES onset was 38.5 years (SD=14.1); mean PNES duration was 7.3 years (SD=6.8). Regarding PNES semiology, five patients reported hypermotor, one reported partial motor, and five reported mixed hyper- and hypomotor/catatonic. (Three PNES patients were subjects in an earlier study regarding a different task [4].)
Seizure-free and neurological symptom-free individuals with “stressful or traumatic life events” were recruited as TCs. TCs were at or above (TC-clin: N=25; women, N=19) or below (TC-nonclin: N=24; women, N=17) a clinical cutoff score of 44 on the PTSD Checklist for DSM-IV–specific event version (PCL-S) (18).
Data on demographic and clinical characteristics that differed by group are presented in Table 1. Compared with TCs, PNES patients were older, less educated, more likely to be married (though not more likely to be in a relationship), and more likely to be taking psychotropic medication. Compared with the TC-nonclin group, PNES patients reported more psychiatric symptoms as measured by the Symptom Checklist–90 Revised (SCL-90) or the Symptom Checklist–53 (SCL-53) (19), with the TC-clin group in between. PCL-S scores were comparable for the PNES and TC-clin groups, with scores for each higher than for the TC-nonclin group. Groups did not differ in gender distribution (mostly women), race-ethnicity (mostly white European-American), household income (mostly lower and lower-middle), or trauma type reported (adult or childhood physical or sexual abuse most frequent).
| PNES (N=11) | TC-clin (N=25) | TC-nonclin (N=24) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | χ2 | df | p | |
| Marital status (married)b | 7 | 63.6a | 7 | 29.2a,b | 4 | 16.7b | 7.88 | 2 | 0.019 | |
| Psychotropic medicationc | 9 | 81.8a | 4 | 16.0b | 3 | 12.5b | 21.03 | 2 | <0.001 | |
| M | SD | M | SD | M | SD | F | df | p | ηp2 | |
| Age (years) | 45.0a | 12.7 | 33.1b | 13.0 | 27.8b | 10.1 | 7.89 | 2, 56 | 0.001 | 0.220 |
| Education (years) | 12.7a | 2.6 | 16.0b | 3.0 | 14.7a,b | 2.3 | 5.94 | 2, 56 | 0.005 | 0.175 |
| PCL-Sd | 49.3a | 20.8 | 53.6a | 8.5 | 34.4b | 8.5 | 18.15 | 2, 56 | <0.001 | 0.393 |
| Symptom Checklist subscalese | ||||||||||
| Global severity index | 1.6a | 1.1 | 1.1a,b | 0.4 | 0.7b | 0.5 | 8.00 | 2, 58 | 0.001 | 0.222 |
| Somatization | 2.0a | 1.0 | 0.8b | 0.5 | 0.6b | 0.5 | 19.12 | 2, 58 | <0.001 | 0.406 |
| Depression | 1.9a | 1.2 | 1.4a,b | 0.7 | 1.1b | 0.8 | 3.62 | 2, 58 | 0.033 | 0.115 |
| Anxiety | 1.9a | 1.3 | 1.1b | 0.6 | 0.5c | 0.5 | 13.17 | 2, 58 | <0.001 | 0.320 |
| Phobic anxiety | 1.4a | 1.4 | 0.6b | 0.6 | 0.2b | 0.2 | 9.98 | 2, 58 | <0.001 | 0.263 |
| Obsessive-compulsive | 2.2a | 1.3 | 1.4b | 0.6 | 1.0b | 0.7 | 8.03 | 2, 58 | 0.001 | 0.223 |
| Psychoticism | 1.1a | 1.0 | 0.7a,b | 0.5 | 0.5b | 0.6 | 3.26 | 2, 58 | 0.046 | 0.104 |
| Paranoia | 1.0a | 1.1 | 1.3a | 0.7 | 0.6b | 0.7 | 4.96 | 2, 58 | 0.010 | 0.151 |
TABLE 1. Demographic and clinical characteristics that differed significantly by study groupa
At the laboratory, participants provided informed consent per American Psychological Association standards, then electrocardiographic sensors were attached for data acquisition. Physiology and video were recorded continuously throughout the session (12, 13), and IBI and RSA were derived offline (Mindware Technologies, Westerville, Ohio). After a 3-minute resting initial baseline, participants rated emotional slides (not reported here) and then were presented with the relived emotions task.
Four trials followed the same procedure: 30-second baseline; instructions; write about event; think/recall event, focusing on feelings; talk or describe aloud (3 minutes); answer questions aloud; 30-second rest. Trial 1 was neutral; participants wrote several sentences about a time they lacked strong feelings, as during a “normal” or “boring” routine. Participants thought about the scenario until picturing it clearly, then pressed a computer key. Subsequent emotion trials (anger, shame, happiness) were counterbalanced; participants indicated when they felt the emotion strongly. Participants were prompted via intercom to describe the event, then rate the difficulty of reliving (0, not at all difficult, to 8, very difficult) and how much target emotion (or, for neutral, how much emotion) was felt during reliving (0, none at all, to 8, most ever).
Data Analysis
Self-report.
We conducted 4 (emotion: neutral, anger, shame, happiness) × 3 (group: PNES, TC-clin, TC-nonclin) analyses of variance (ANOVAs; emotion condition within-subjects, group between-subjects) for relived emotion task difficulty and amount of target emotion during reliving.
Cardiovascular.
We examined change from the pretask baseline in IBI and RSA when thinking about, talking about, and resting after the memory, using similarly structured ANOVAs, including epoch (think, talk, rest) as an additional repeated measure.
Control variables.
Given that group differences in age, education, and comorbid psychiatric symptoms (Global Symptom Inventory [GSI] scores) might influence results, we added them (mean-centered) and their interactions with group to each analysis (i.e., control variables). To preserve degrees of freedom, we omitted age-, education-, and GSI-by-group interaction terms if nonsignificant.
All follow-up, pairwise comparisons were Bonferroni corrected. Partial eta-squared (ηp2) indicated effect size. Significance tests were two-tailed.
Results
The relived emotion induction was effective, based on events recalled and higher target emotion ratings across emotion conditions (mean=5.9 [SD=1.2]) versus in the neutral condition (mean=2.8, [SD=1.9]).
Task Engagement
All TCs completed all tasks; however, three of the 11 PNES patients did not complete the relived shame task (χ2=14.07, df=2, p=0.001, N=60). One refused (“too difficult”), one could not generate anything even with prompting, and one completed the writing portion then stopped. Each of these three individuals subsequently had a PNES episode. Notably, these reactions occurred in the two condition orders where shame was last.
Another PNES participant would not describe or rate anger (only “frustration” and “annoyance”). One participant had a PNES episode while painstakingly describing a neutral event. All PNES participants relived happy events without incident.
Task Difficulty
The emotion effect was significant (F=33.80, df=3, 52, p<0.001, ηp2=0.661) (Figure 1A). Difficulty ratings were higher for reliving anger and shame, compared with happiness or a neutral event. The group effect was significant (F=7.89, df=2, 54, p=0.001, ηp2=0.226). Those with PNES reported greater difficulty reliving emotions (mean=4.3 [SE=0.4]) than those in the TC-nonclin group (mean=2.3 [SE=0.2]; p=0.001). PNES and TC-clin (mean=3.1 [SE=0.2]) did not differ (p=0.059). The interaction of emotion and group was not significant (F=1.64, df=6, 106, p=0.144, ηp2=0.085).
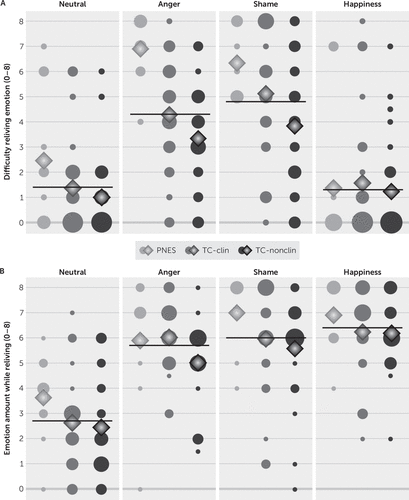
FIGURE 1. Self-reported difficulty and amount of emotion during a relived emotion task, by group and emotion conditiona
a Panel A shows the extent of difficulty reliving emotion among patients with psychogenic nonepileptic seizures (PNES) and trauma control subjects (TCs) with posttraumatic stress symptoms at or above (TC-clin) versus below (TC-nonclin) a clinical cutoff score on the PTSD Checklist for DSM-IV–specific event version (PCL-S). Participants were prompted via intercom to describe the event, then rate the difficulty of reliving (panel A) on a scale from 0 to 8 (0=not at all difficult, 8=very difficult). Panel B shows the amount of target emotion participants experienced during reliving (0=none at all, 8=most ever); for the neutral condition, participants rated “how much emotion” they felt in general while reliving. Circles represent the numbers of participants with a given value on the dependent variable (DV); the smallest circle in each panel represents one participant. Diamonds represent the means of the DV by group within emotions. Horizontal lines represent the overall means of the DV across groups within emotions.
Including control variables, emotion and group effects remained significant. In addition, an interaction of emotion and group emerged (F=2.65, df=6, 98, p=0.020, ηp2=0.140); PNES reported greater difficulty reliving anger than either TC-nonclin (p<0.001) or TC-clin (p=0.007).
Target Emotion Ratings
The emotion effect was significant (F=50.04, df=3, 52, p<0.001, ηp2=0.743) (Figure 1B). Participants reported experiencing more of the target emotion in the anger, shame, and happiness conditions than in the neutral condition (p<0.001 for all), and more of the target emotion in the happiness condition than in the anger condition (p=0.007). The effect of group was not significant (F=3.08, df=2, 54, p=0.054, ηp2=0.102), nor was the interaction of emotion and group (F=0.87, df=6, 106, p=0.522, ηp2=0.047). When control variables were included, emotion effects remained significant.
Cardiovascular Physiology
Except for the initial baseline, all IBI and RSA analyses used change scores (Δ) from the immediate pretask baseline in the relevant emotion trial. For IBI, all effects were nonsignificant, except the expected increased heart rate when speaking (reflected by a significant epoch effect); IBI results are omitted here for brevity.
One-way ANOVA on initial baseline revealed no group difference in RSA (F=1.67, df=2, 55, p=0.197, ηp2=0.057; PNES, mean=5.1 [SE=0.4]; TC-clin, mean=5.8 [SE=0.3]; TC-nonclin, mean=6.1 [SE=0.3]). For ΔRSA, the 4 (emotion) × 3 (epoch) × 3 (group) interaction was significant (F=2.03, df=12, 74, p=0.034, ηp2=0.247); emotion × epoch and emotion × group were also significant.
For happiness, ΔRSA differed by group (F=8.22, df=2, 49, p=0.001, ηp2=0.251). During think, talk, and rest, the PNES and TC-clin groups both showed RSA decreases (PNES, mean=–0.67 [SE=0.27]; TC-clin, mean=–0.24 [SE=0.18]; PNES versus TC-clin, p=0.569), whereas the TC-nonclin group showed RSA increases (mean=0.56 [SE=0.19]) (PNES versus TC-nonclin, p=0.002; TC-clin versus TC-nonclin, p=0.012). Neither epoch (F=1.29, df=2, 48, p=0.284, ηp2=0.051) nor epoch × group (F=1.98, df=4, 98, p=0.103, ηp2=0.075) was significant.
When control variables were included in analyses of ΔRSA during relived happiness, group remained significant. An epoch × group effect also emerged (F=2.50, df=4, 90, p=0.048, ηp2=0.100). During think, there were no group differences (p>0.104 for all); during talk, PNES and TC-clin showed similar RSA decreases (p=0.614), whereas TC-nonclin showed increases (PNES versus TC-nonclin, p=0.012; TC-clin versus TC-nonclin, p=0.038); and during rest, PNES showed greater decreases than either TC-clin (p=0.007) or TC-nonclin (p<0.001).
For anger, epoch was significant (F=3.94, df=2, 49, p=0.026, ηp2=0.138). RSA decreased during think and talk and increased during rest; however, the epoch effect became nonsignificant when control variables were included. Neither group (F=1.21, df=2, 50, p=0.306, ηp2=0.046) nor epoch × group (F=1.98, df=4, 100, p=0.103, ηp2=0.074) was significant.
For neutral and shame, ΔRSA effects were nonsignificant (F≤1.03 for all, p≥0.364 for all, ηp2≤0.042 for all).
Discussion
Groups surprisingly did not differ in the amount of target emotion reported during reliving; however, those with PNES reported greater overall difficulty reliving both neutral and emotional events. Furthermore, three PNES patients did not relive shame, whereas all TCs did so. RSA findings during and after relived happiness showed decreases for those in the PNES and TC-clin groups, compared with increases for those in the TC-nonclin group.
Results fit with clinical observations that for those with PNES, cognitive and emotional engagement feels more effortful (20) and, in fact, may require more effort (21). If engagement is too difficult, emotion avoidance may arise as a coping strategy (3, 5, 7). When controlling for demographic and clinical differences, individuals with PNES reported particular difficulty reliving anger (more than either TC group), consistent with prior research (1, 2). Notably, instances of shame avoidance occurred when shame was the final emotion condition, suggesting that an “accumulation” of emotion and/or of regulatory effort may have contributed. Difficulty categorizing internal emotional states and regulating high arousal may lead to ongoing (possibly automatic) overregulation attempts; profound avoidance in the form of a PNES or dissociative episode may be part of these attempts, or may result if other regulation fails. Thus, confrontation with emotion-inducing events (internal or external), or even the possibility of encountering such events, potentially initiates a cascade of neurobiological processes that proximally trigger PNES episodes and create vulnerabilities for future episodes (5, 6, 9). Trajectories of processing shame experiences may reveal a particular vulnerability, with extent of avoidance in these contexts differentiating individuals with PNES and posttraumatic stress symptoms from individuals with only posttraumatic stress symptoms.
RSA typically increases with relaxation and decreases when demands exceed perceived resources (22). Thus, RSA reductions for the PNES and TC-clin groups during and after relived happiness suggest that “feeling happy” was more difficult or less relaxing than it was for the TC-nonclin group, whose RSA increased. Given similarities between the PNES and TC-clin groups, traumatic stress likely contributes. Effects may be longer lasting for those with PNES, given that the PNES group showed greater RSA decreases during posttask rest than did the TC-clin group when demographic and clinical differences were statistically controlled. We speculate that interoceptive awareness and emotion categorization are less compromised in the TC-clin group, and, therefore, motor and other PNES symptoms do not develop; both populations may have similar traumatic experiences, dissociative tendencies, and regulatory deficits, but we suggest that their effects are more heterogeneous and far reaching in PNES.
Results should be replicated in a larger sample. Our study was underpowered, increasing the possibility of type II error and limiting generalizability. Group sizes were unequal (TC groups each were double the size of the PNES group), and groups were not fully matched. With such a small PNES sample, it was not feasible to examine effects of marital status and medication use, although some findings (e.g., greater avoidance) were similar to previous studies with unmedicated PNES samples (2). Patterns in our data were fairly consistent within the PNES group. Nevertheless, previous studies have identified PNES subgroups (23, 24); potential differences among their reactions to relived emotions may be informative. Quantifying what other emotions and bodily sensations participants felt besides the target emotions and adding observational coding of microfacial expressions are important next steps.
Conclusions
Explanations regarding potential mechanisms of emotional overwhelm in PNES and their neurobiological underpinnings provide scientific understanding to help patients and their families understand that PNES is a real and treatable condition; this understanding in turn facilitates treatment acceptance and recovery (25). Although preliminary, this study offers a window into subjective and physiological reactions to specific emotional provocations, ultimately aiming to bridge the gaps among distal causes, such as childhood trauma, proximal external and internal emotion triggers, and PNES symptoms.
1 : Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia 2009; 50:1001–1011Crossref, Medline, Google Scholar
2 : Automatic avoidance tendencies in patients with psychogenic non-epileptic seizures. Seizure 2011; 20:628–634Crossref, Medline, Google Scholar
3 : Anxiety and avoidance in psychogenic nonepileptic seizures: the role of implicit and explicit anxiety. Epilepsy Behav 2014; 33:77–86Crossref, Medline, Google Scholar
4 : Emotion in psychogenic nonepileptic seizures: responses to affective pictures. Epilepsy Behav 2012; 24:107–115Crossref, Medline, Google Scholar
5 : Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry 2014; 71:52–60Crossref, Medline, Google Scholar
6 : Functional connectivity of dissociation in patients with psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry 2012; 83:239–247Crossref, Medline, Google Scholar
7 : Towards an integrative theory of psychogenic non-epileptic seizures (PNES). Clin Psychol Rev 2016; 47:55–70Crossref, Medline, Google Scholar
8 : The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci 2013; 14:143–152Crossref, Medline, Google Scholar
9 : An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clin EEG Neurosci 2015; 46:4–15Crossref, Medline, Google Scholar
10 : Alterations of consciousness in psychogenic nonepileptic seizures: emotion, emotion regulation and dissociation. Epilepsy Behav 2014; 30:43–49Crossref, Medline, Google Scholar
11 : Autonomic nervous system functioning associated with psychogenic nonepileptic seizures: analysis of heart rate variability. Epilepsy Behav 2016; 54:14–19Crossref, Medline, Google Scholar
12 : Emotion, physiology, and expression in old age. Psychol Aging 1991; 6:28–35Crossref, Medline, Google Scholar
13 : Effects of posttraumatic stress disorder status and covert hostility on cardiovascular responses to relived anger in women with and without PTSD. Biol Psychol 2009; 82:274–280Crossref, Medline, Google Scholar
14 Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013Google Scholar
15 : Autobiographical memory for shame or guilt provoking events: association with psychological symptoms. Behav Res Ther 2010; 48:646–652Crossref, Medline, Google Scholar
16 : Cognitive differences between patients who have psychogenic nonepileptic seizures (PNESs) and posttraumatic stress disorder (PTSD) and patients who have PNESs without PTSD. Epilepsy Behav 2014; 37:82–86Crossref, Medline, Google Scholar
17 : Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. J Neurol Neurosurg Psychiatry 2017; 88:491–497Crossref, Medline, Google Scholar
18 : Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 1996; 34:669–673Crossref, Medline, Google Scholar
19 : SCL-90-R, Brief Symptom Inventory, and matching clinical rating scales; in The Use of Psychological Testing for Treatment Planning and Outcome Assessment. Edited by Maruish ME. Hillsdale, NJ, Erlbaum, 1994Google Scholar
20 : Mechanisms of possible neurocognitive dysfunction, in Psychogenic Nonepileptic Seizures: Toward the Integration of Care. Edited by Dworetzky BA, Baslet GC. New York, Oxford University Press, 2017Google Scholar
21 : Cognitive deficits and emotion regulation strategies in patients with psychogenic nonepileptic seizures: a task-switching study. Epilepsy Behav 2014; 32:108–113Crossref, Medline, Google Scholar
22 : Cardiac vagal control as a marker of emotion regulation in healthy adults: a review. Biol Psychol 2017; 130:54–66Crossref, Medline, Google Scholar
23 : Emotional dysregulation, alexithymia, and attachment in psychogenic nonepileptic seizures. Epilepsy Behav 2013; 29:178–183Crossref, Medline, Google Scholar
24 : Autonomic and subjective responsivity to emotional images in people with dissociative seizures. J Neuropsychol 2018; 12:341–355Crossref, Medline, Google Scholar
25 : Psychogenic non-epileptic seizures in children and adolescents, part II: explanations to families, treatment, and group outcomes. Clin Child Psychol Psychiatry 2018; 23:160–176Crossref, Medline, Google Scholar



