Autonomic, Endocrine, and Inflammation Profiles in Functional Neurological Disorder: A Systematic Review and Meta-Analysis
Abstract
Objective:
Functional neurological disorder (FND) is a core neuropsychiatric condition. To date, promising yet inconsistently identified neural circuit profiles have been observed in patients with FND, suggesting that gaps remain in our systems-level neurobiological understanding. As such, other important physiological variables, including autonomic, endocrine, and inflammation findings, need to be contextualized for a more complete mechanistic picture.
Methods:
The investigators conducted a systematic review and meta-analysis of available case-control and cohort studies of FND. PubMed, PsycINFO, and Embase databases were searched for studies from January 1, 1900, to September 1, 2020, that investigated autonomic, endocrine, and inflammation markers in patients with FND. Sixty-six of 2,056 screened records were included in the review, representing 1,699 patients; data from 20 articles were used in the meta-analysis.
Results:
Findings revealed that children and adolescents with FND, compared with healthy control subjects (HCs), have increased resting heart rate (HR); there is also a tendency toward reduced resting HR variability in patients with FND across the lifespan compared with HCs. In adults, peri-ictal HR differentiated patients with functional seizures from those with epileptic seizures. Other autonomic and endocrine profiles for patients with FND were heterogeneous, with several studies highlighting the importance of individual differences.
Conclusions:
Inflammation research in FND remains in its early stages. Moving forward, there is a need for the use of larger sample sizes to consider the complex interplay between functional neurological symptoms and behavioral, psychological, autonomic, endocrine, inflammation, neuroimaging, and epigenetic/genetic data. More research is also needed to determine whether FND is mechanistically (and etiologically) similar or distinct across phenotypes.
Functional neurological disorder (FND) is a condition at the intersection of neurology and psychiatry (1). While of interest to early clinical neuroscience leaders, such as Jean-Martin Charcot and Sigmund Freud, FND was neglected by clinicians and academics alike throughout much of the late 20th century. Nonetheless, FND is the second most common condition seen in outpatient neurology clinics, a finding compounded by observations of high health care costs and poor prognoses among many patients (2–4). Renewed interest in the field has been promoted by recognition that FND can be reliably diagnosed using physical examination signs and semiological features (1, 5). This high diagnostic specificity has led to a surge in research on the pathophysiology of FND, particularly using brain imaging approaches (6, 7). Promising yet inconsistently identified neural circuit profiles have been observed to date (6), suggesting that there are important gaps in our systems-level understanding of this condition. Thus, there is a need to contextualize autonomic, endocrine, and inflammation findings for a more complete mechanistic understanding of FND. Such efforts offer the promise of developing novel biologically-informed treatments.
Some functional neuroimaging studies across the motor spectrum of FND (including functional seizures [FND-seiz]) have identified several noteworthy findings in patients compared with healthy control subjects (HCs): enhanced amygdala reactivity in response to affectively valenced stimuli (8, 9), increased task and resting-state connectivity between salience network and motor control circuits (9–11), and hypoactivation and altered connectivity of the right temporoparietal junction and inferior parietal lobule (12–14). Additionally, resting-state functional connectivity studies have found positive correlations between salience network and motor control network connectivity strength and patient-reported symptom severity (11, 15). However, findings have been inconsistent across studies. For example, not all FND cohort studies have observed increased coupling between salience and motor control networks (16).
Several gray and white matter characterization studies in FND samples have also implicated brain networks similar to those identified using functional neuroimaging, including two studies reporting that decreased white matter integrity of the stria terminalis and fornix (an amygdala outflow tract) correlated with FND illness duration and age at onset (17, 18). Nonetheless, structural neuroimaging findings have also been inconsistently identified across studies (6). Thus, while the neuroimaging literature suggests that some patients with FND have functional and structural alterations in brain areas implicated in emotion and threat processing, salience, arousal, agency, and motor control, the observed heterogeneity limits conclusions about specific FND neural signatures. This may relate to differences in disease-related mechanisms across patients, phenotypic heterogeneity, concurrently present neuropsychiatric disorders, medication effects, or compensatory neuroplastic effects.
Given salience and limbic network involvement in some patients with FND, characterizing sympathetic or parasympathetic activity (e.g., heart rate [HR], HR variability [HRV], and skin conductance; Table 1) and endocrine hypothalamic-pituitary-adrenal (HPA) axis markers are important gaps in the literature. Similarly, quantifying inflammation profiles, which are known to modulate salience and limbic networks (19, 20), would add mechanistic value. In the present study, we conducted a systematic review and meta-analysis to comprehensively characterize the available literature on the autonomic, endocrine, and inflammation profiles of patients with FND. In addition, we contextualized this literature with other available pathophysiology considerations.
| Parameter | Definition |
|---|---|
| General parameters | Heartbeat fluctuations irrespective of autonomic innervation |
| TP | The sum of HF and LF |
| R-R or interbeat intervals | The time elapsed between consecutive heartbeats |
| SDNN | The R-R intervals of normal heartbeats; ectopic beats are excluded |
| Parasympathetic parameters | Heart rate changes mediated by vagal (parasympathetic) tone |
| rMSSD | An index of parasympathetic tone less prone to respiratory changes |
| HF or respiratory sinus arrythmia | Heart rate changes that reflect the respiratory cycle patterns |
| CVI | A measure of HF with less physiological noise |
| Sympathetic parameters | Heart rate changes associated with sympathetic activity |
| LF | A measure predominantly driven by the sympathetic nervous system |
| CSI | An LF-related measure with less physiological noise |
TABLE 1. Heart rate variability parameter definitionsa
METHODS
Search Strategy and Selection Criteria
We followed PRISMA guidelines for performing a systematic review and meta-analysis and registered the study in PROSPERO (identification number CRD42020157679). The databases PubMed, PsycINFO and Embase were searched from January 1, 1900, to September 1, 2020, using the following search terms: (“functional neurological disorder” OR “functional neurological symptom disorder” OR “conversion disorder” OR “psychogenic” OR “pseudoseizure” OR “non-epileptic” OR “dissociative seizures” OR “hysteri*” OR “non-organic”) AND (“heart rate” OR “heart rate variability” OR “electrodermal” OR “skin conductance” OR “autonomic” OR “sympathetic” OR “parasympathetic” OR “neuroendocrine” OR “hormone” OR “cortisol” OR “HPA” OR “hypothalamic pituitary adrenal” OR “amylase” OR “brain-derived neurotrophic factor” OR “BDNF” OR “glucocorticoid” OR “inflammat*” OR “interleukin-*” OR “immune” OR “autoimmun*” OR “innate immun*” OR “TNF” OR “C reactive protein” OR “erythrocyte sedimentation rate” OR “lactate” OR “anion gap”). Additional potentially eligible articles were identified from those known to the authors and following inspection of reference lists.
Inclusion criteria were as follows: case-control or cohort studies with at least five adult or pediatric participants with FND who had autonomic, endocrine, or inflammation data points measured. Case reports, abstracts, dissertations, review articles, and papers written in a language other than English were excluded. Studies that exclusively measured imaging, electroencephalographic, or prolactin data were also excluded and previously reviewed elsewhere (5, 6, 21); published articles on somatic symptom disorders, somatoform pain, somatization disorder, undifferentiated somatoform disorder, and the spectrum of functional somatic disorders (e.g., fibromyalgia) were also excluded.
Data Extraction and Systematic Review
EndNote was used to compile abstracts. After removing duplicates, two researchers (S.P.E. and J.M.) independently applied inclusion and exclusion criteria to determine articles to be read. Discrepancies between the two reviewers were resolved by another researcher (D.L.P). Data regarding demographic characteristics, outcome measurements, and results presented in the text, tables, or graphs were systematically extracted and reviewed. Quality and bias were assessed using the National Institutes of Health Study Quality Assessment Tools guidelines from the National Heart, Lung and Blood Institute for cohort and case-control studies (22). All eligible articles were included in the systematic review.
Meta-Analysis
At least four independent articles had to report findings on a given autonomic, endocrine, or inflammation finding to be pooled and added in the meta-analysis. Because only a limited number of studies recorded these data in FND populations, we used a transdiagnostic approach across FND subtypes. Authors were contacted when data were not explicitly reported as measures of central tendency or dispersion; in three instances, between-group comparisons (i.e., Student’s t test) were performed when absent in the article (23–25). We followed Cochrane guidelines for data preparation.
For all meta-analyses, we used the software Stata/IC 16.0 (StataCorp, College Station, Tex.). With the command meta, we calculated effect sizes (θ): mean difference for raw data and the Hedge’s g standardized mean difference for transformed data. To measure precision, we used 95% confidence intervals with unequal variances for mean difference comparisons. All estimations were calculated with the random-effects model after testing homogeneity (Q), heterogeneity (H2), and variation (I2). Between-study variability (τ2) was computed with the restricted maximum likelihood (REML) model and used to calculate the weight of every primary study. Analyses on peri-ictal time points (preictal, ictal, and postictal) in FND-seiz studies were also performed. Funnel plots and sensitivity analyses evaluated publication bias and estimated the effects of individual studies.
RESULTS
Sixty-six articles, as described in the PRISMA flow diagram (Figure 1), were included in the systematic review, with 20 articles included in the meta-analysis. In general, most studies reported clear research questions and inclusion-exclusion criteria but were typically not blinded to group identity, and sample sizes were small. Additionally, more recently published studies adhered better to quality standards. For further details on the study quality, see Table S1 in the online supplement; for funnel plot and sensitivity analysis results, see Figures S1–S7 in the online supplement.
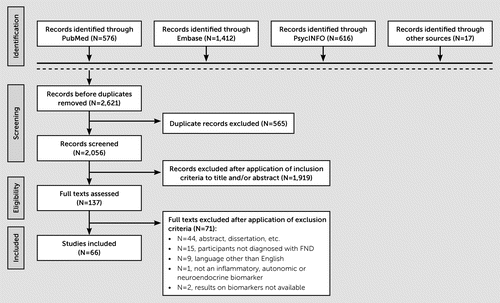
FIGURE 1. Flow diagram of article selectiona
a FND=functional neurological disorder.
Autonomic Findings
Baseline HR
In eight articles, baseline mean HR was measured in patients with FND compared with HCs (26–30), with normative data (31), or with neurological populations (32, 33). In adults, baseline HR findings have been inconsistent. Compared with HCs, FND-seiz (N=20) and functional movement disorder (FND-movt; N=20) samples did not show group-level resting-HR differences (30); however, patients with FND-movt (N=35) exhibited a higher baseline HR compared with HCs in another study (29). Across two pediatric cohorts (mixed FND and FND-seiz), baseline HR was increased in patients compared with HCs (26–28). In a third pediatric FND-seiz sample (N=33), 18% of patients had a baseline HR in the 90th percentile of expected norms, a finding that correlated with seizure frequency (31). Additionally, there were no baseline HR differences in nine patients with mixed FND compared with neurological control subjects (e.g., cervical lesion) (32) or between patients with functional syncope (N=44) compared with neural-mediated syncope (N=44) (33).
When applying a meta-analysis to the four FND studies (across five cohorts; one study reported FND-movt and FND-seiz data separately), compared with HCs, there was no statistically significant effect of group on baseline mean HR (mean difference=5.08, 95% CI=−0.87, 11.03; Figure 2). However, based on the sensitivity analysis (see Figure S1 in the online supplement), removal of the Demartini et al. (30) FND-seiz cohort would result in a statistically significant effect of FND group identity on increased baseline mean HR (mean difference=7.43, 95% CI=2.69, 12.17; Figure 2).
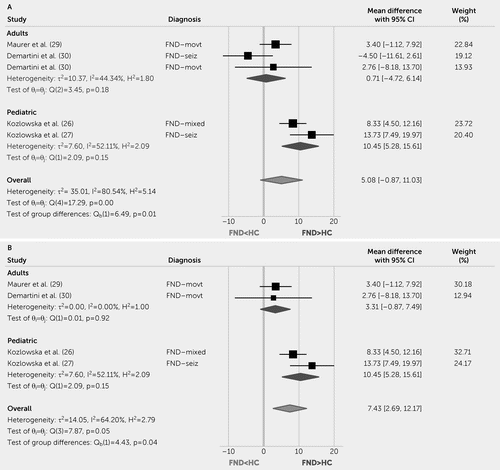
FIGURE 2. Forest plot of baseline heart rate mean difference between patients with functional neurological disorder (FND) compared with healthy control (HC) subjectsa
a Panel A shows a forest plot of the baseline heart rate mean difference between the FND and HC cohorts. Panel B shows a forest plot of the baseline heart rate mean difference between the FND and healthy control cohorts, when the functional seizures (FND-seiz) cohort in the Demartini et al. study (30) is removed based on the sensitivity analysis results, see Figure S1 in the online supplement. θ=effect size; FND-mixed=mixed FND; FND-movt=functional movement disorder; H2=heterogeneity; I2=variation; Q=homogeneity; REML=restricted maximum likelihood model; τ2=variability.
Task-related HR
Five studies measured mean HR in patients with FND compared with HCs during task performance (26, 32, 34–36). Across cognitive and affective paradigms (i.e., Stroop, emotional Stroop, and low- and high-stress mathematics), two FND-seiz cohorts showed no group-level differences in task-related HR (34, 35), although patients with FND-seiz reporting higher levels of perceived stress exhibited smaller HR changes (35). Conversely, another study identified that patients with FND-seiz showed a shorter HR deacceleration amplitude compared with HCs during visual emotion processing (36). Compared with neurological control subjects, no task-related HR differences were observed in patients with mixed FND symptoms during several tasks (i.e., illness interview, word-association task, and presentation of physical stressors) (32). In a mixed pediatric FND cohort, higher HR was observed during three tasks (i.e., auditory oddball, Go/No-go, and facial emotion-perception) in patients compared with HCs (26); additionally, HCs showed greater HR increases during auditory oddball and Go/No-go tasks compared with pediatric FND patients (26).
Peri-ictal or event-related HR
Five FND-seiz studies measured mean HR during preictal, ictal, and postictal periods (23, 37–40). Compared with patients with epileptic seizures (23, 37–39), studies showed a lower HR in patients with FND-seiz preictally (23, 37), ictally (23, 37, 39), and postictally (23, 37, 38). Additionally, a within-group study found higher preictal and decreased postictal HR in FND-seiz patients relative to their baseline HR (40). In 66 symptomatic children and adolescents with mixed FND, increased mean HRs were observed compared with HCs (41).
In a meta-analysis of case-control studies (23, 37–39), patients with FND-seiz were more likely to have a lower mean HR than individuals with epileptic seizures ictally and postictally (mean difference=−21.72, 95% CI=−22.77, −20.66 and mean difference=−12.47, 95% CI=−20.01, −4.94, respectively; Figure 3). Considering all peri-ictal time points, patients with FND-seiz also showed a significant overall lower mean HR (mean difference=−12.65, 95% CI=−20.20, −5.11). For meta-analysis findings related to within-group peri-ictal comparisons in patients with FND-seiz, see Figure S8 in the online supplement.
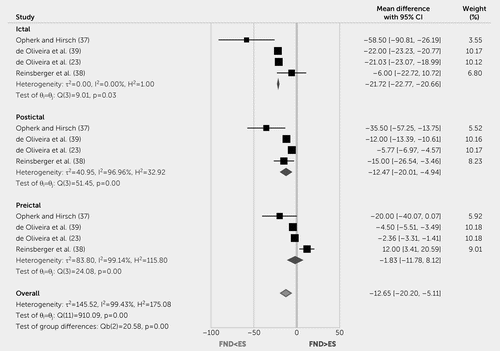
FIGURE 3. Forest plot of peri-ictal heart rate mean difference between patients with functional seizures versus epileptic seizuresa
a θ=effect size; ES=epileptic seizures; FND=functional neurological disorder; H2=heterogeneity; I2=variation; Q=homogeneity; REML=restricted maximum likelihood model; τ2=variability.
Tilt Table Findings
By using tilt table testing, one study observed that patients with functional syncope had significantly higher mean HR 120 seconds prior to losing consciousness relative to those with neurally mediated syncope (33); notably, this difference became more pronounced at 30 seconds prior to loss of consciousness, where the HR began to drop in participants with neurally mediated syncope and those with functional syncope continued to show escalating HRs (33). Similarly, a within-group study found that eight patients with functional syncope showed rising HRs immediately preceding loss of consciousness (42).
Summary of HR Findings
Evidence suggests that patients with pediatric FND have elevated baseline HRs compared with HCs. The study of HR changes during task performance has not robustly identified group-level differences to date. Compared with epileptic seizures, patients with FND-seiz show decreased HRs ictally and postictally (a finding confirmed by our meta-analysis). HR profiles during tilt table testing may aid the differentiation of functional versus other causes of syncope.
General HRV Parameters
Across studies, results on total power (24, 29, 43), R-R (interbeat) interval variability (24, 43–47), and standard deviation of normal to normal intervals (24, 29, 43, 45, 48) are inconsistent. To date, these markers do not reliably differentiate between patients with FND, patients with epileptic seizures, and HCs during any time points.
HRV Parasympathetic Parameters
Eight FND studies reported the root mean square of successive differences (rMSSD) in FND patients compared with HCs (26, 29, 34, 41, 43) and/or with individuals with epileptic seizures (24, 43, 48, 49). At rest, lower rMSSD was identified across a variety of FND cohorts compared with HCs (26, 29, 34, 43); however, this parameter did not differentiate FND-seiz cohorts compared with epileptic seizure cohorts (24, 43, 48, 49). Pediatric studies found that patients with mixed FND had lower rMSSD during cognitive-affective tasks (26) and while symptomatic, compared with HCs (41). One study found increased rMSSD in FND-seiz patients during events compared with patients with epileptic seizures (24), while another study did not find any group-level ictal differences (49).
In the meta-analysis between the four case-control studies comparing FND cohorts and HCs at baseline, there was no significant effect of diagnosis on resting rMSSD (standardized mean difference=−0.28, 95% CI=−0.64, 0.09; Figure 4).
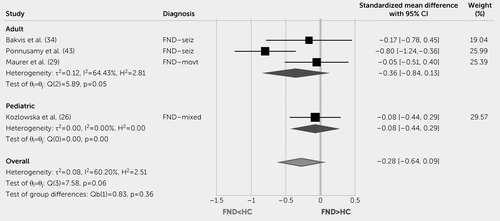
FIGURE 4. Forest plot of baseline heart rate variability (rMSSD) standardized mean difference between patients with functional neurological disorder (FND) compared with healthy control (HC) subjectsa
a θ=effect size; FND-mixed=mixed FND; FND-movt=functional movement disorder; functional seizures (FND-seiz); H2=heterogeneity; HC=healthy control subjects; I2=variation; Q=homogeneity; REML=restricted maximum likelihood model; rMSSD=root mean square of successive differences; τ2=variability.
Eight studies described high frequency (HF) in FND patients compared with HCs (26, 29, 43, 46, 47), patients with epileptic seizures (24, 43, 49), patients mixed FND-seiz/epileptic seizures (45), and/or trauma control subjects (46, 47). Compared with HCs, studies reported decreased resting HF in mixed FND (26) and FND-seiz (43), while a separate FND-movt study did not find any group-level differences (29). During behavioral/cognitive tasks, decreased HF was observed while recalling happy memories (46) and performing auditory oddball and facial recognition tasks (26) in patients with FND-seiz compared with HCs; however, no group-level differences were observed during Go/No-go (26) and affective picture-viewing tasks (47). Three studies did not find differences in resting HF between patients with FND-seiz and those with epileptic seizures (24, 43, 49). However, ictal HF findings in FND-seiz patients compared with epileptic seizure patients have been inconsistent (24, 49). There were also no resting or peri-ictal HF differences in FND-seiz patients compared with FND-seiz/epileptic seizure patients (45).
One study identified that patients with FND-seiz showed a higher resting cardiovagal index compared with HCs (43); however, this measurement did not reliably differentiate patients with FND-seiz from epileptic seizure(24, 43, 48, 49) or FND-seiz/epileptic seizure(45) cohorts across rest and/or peri-ictal periods.
HRV Sympathetic Parameters
Five studies recorded low frequency (LF) in FND populations (24, 29, 31, 43, 45). An FND-seiz pediatric cohort had 0.6% LF at baseline (31). Two studies compared findings with HCs: one identified decreased LF in FND-seiz at rest (43); however, there were no group differences observed in a FND-movt cohort (29). Compared with epileptic seizures, two studies did not find resting LF differences with FND-seiz (24, 43), but one reported higher LF in the FND-seiz group ictally (24). A study of 11 FND-seiz patients compared with 11 patients with FND-seiz/epileptic seizure found that those with FND-seiz only had higher LF irrespective of condition (baseline, preictal, ictal, or postictal) (45).
Five studies reported the cardio sympathetic index (CSI) of participants (24, 43, 45, 48, 49). At baseline, patients with FND-seiz had a higher CSI than HCs (43), and all but one study (48) reported a similar CSI with epileptic seizures (24, 43, 49) and FND-seiz/epileptic seizure patients (45). Ictally, two studies showed that patients with FND-seiz had a decreased ictal CSI compared with patients with epileptic seizures (24, 49), and one reported a similar CSI with FND-seiz/epileptic seizure patients (45).
Summary of HRV Findings
Across resting and task conditions, children and adolescents with FND showed lower rMSSD (indicative of reduced parasympathetic tone) compared with HCs. There is also a tendency toward reduced rMSSD in adults with FND compared with HCs. Other indices of HRV have not robustly differentiated FND patients compared with HCs or with patients with epileptic seizures across studies.
Skin Conductance (Electrodermal) Activity (SCA)
Three studies compared SCA between patients with FND and HCs (28, 50) or epileptic seizures (51). Compared with HCs, children and adolescents with mixed FND had increased baseline SCA (28), while adults with mixed FND did not show resting group-level differences (50). Compared with patients with epileptic seizures, patients with FND-seiz had decreased SCA during the peri-ictal period (51).
Skin Conductance Level (SCL)
Five studies measured SCL at baseline (52), during baseline and task (31, 53–55), or only at task (56). In the article that reported findings at baseline only, nine mixed FND patients had more SCL fluctuations compared with HCs (52). At baseline and task, three case-control studies reported varied results (53–55): 40 FND-seiz patients had increased resting SCL compared with HCs after controlling for depression and antidepressant use, with no group differences during a facial affect recognition task (53). At baseline and while viewing affectively valenced images, the SCL of 39 patients with FND-seiz covaried with depression scores, but there were no group differences with HCs when controlling for depression (54). Additionally, during interoceptive accuracy and dissociation-induction tasks, there were no SCL differences between mixed FND patients and HCs (55). Meanwhile, in a pediatric sample, SCL failed to return to baseline levels following a cognitive stress task in 18 of 31 patients with FND-seiz, a finding that correlated with greater illness duration (31). In the study that reported task SCL only, patients with mixed FND had significantly higher SCL fluctuations than HCs and psychiatric control subjects during an auditory task (56). The meta-analysis of the four case-control studies did not reveal a significant effect of group on SCL during task performance (standardized mean difference=−1.82, 95% CI=−1.40, 5.03; see Figure S9 in the online supplement).
Skin Conductance Response (SCR)
Nine studies reported SCR findings at baseline (44, 51), peri-ictally (44, 51), and/or during a trigger or task (32, 36, 53, 54, 57–59). Interictally, one study showed that patients with FND-seiz had decreased upper-limb SCR compared with patients with epileptic seizures (44), while another did not reveal group differences (51). Yet, investigators in both studies observed that peri-ictally, individuals with epileptic seizures had significantly greater SCR than patients with FND-seiz (44, 51). Compared with patients with peripheral neuropathies, one study reported that only patients with functional sensory symptoms elicited an SCR to a pinprick (58). In contrast, another study revealed that mixed FND patients had increased SCR in response to specific interview questions and threat-related words, but no overall group differences were observed (32).
Although there were no group differences between patients with FND-movt and HCs during an auditory stimulus (59), studies comparing SCR between FND-seiz patients and HCs reported differentiating task-related findings (36, 53, 54, 57). Two studies showed that while viewing affectively valenced images (36) or films (57), patients with FND-seiz had lower SCR than HCs, a potential marker of decreased (or suppressed) emotion processing in the patient group. Interestingly, in the subgroup of participants with a heightened autonomic response, researchers found that patients with FND-seiz compared with HCs had increased SCR while viewing affectively valenced images (54) and decreased SCR amplitude during a facial emotion recognition task (53).
Skin Conductance Habituation Rate
Three studies investigated skin conductance habituation rates (56, 59, 60). A case-control study found that patients with FND-movt compared with HCs had similar habituation to an auditory stimulus (59); another study reported that, unlike psychiatric and HCs, mixed FND patients exhibited impaired habituation after a trigger (56). Finally, one study found that trait anxiety scores were closely linked to habituation rate variance in 10 patients with mixed FND (60).
Startle Response
Two studies compared acoustic startle responses in patients with FND-movt compared with HCs (59, 61). One found that patients had increased early (physiological) and late (behavioral) startle response probability (59), while the other did not find group differences in startle-response latency or amplitude (61).
Summary of Skin Conductance and Startle Responses
Overall, SCL, SCR, and startle response in modest sample size studies have demonstrated mixed results, suggesting heterogeneity at rest and during task performance across patients with FND. Early indications suggest that SCRs are decreased peri-ictally in patients with FND-seiz compared with epileptic seizures.
Endocrine Findings
Cortisol
Eleven studies measured baseline cortisol levels, with measurement approaches varying across studies (25, 48, 62–71). There is near consensus that FND patients have similar baseline cortisol levels compared with HCs (62–69). In terms of diurnal patterns, six studies did not find group-by-time differences between patients with mixed FND (69), FND-movt (62, 65), or FND-seiz (63, 64, 66, 67) compared with HCs; one study found between-group differences from 12:00 p.m. to 8:00 p.m. (68) in patients with FND-seiz compared with HCs. Notably, the post hoc analysis of the latter study found that higher salivary cortisol levels in patients with FND-seiz were driven by those who reported past sexual trauma (68). Our meta-analysis showed that compared with HCs, there was no consistent effect of FND on baseline cortisol levels (standardized mean difference=0.47, 95% CI=−0.47, 1.42; Figure 5A).
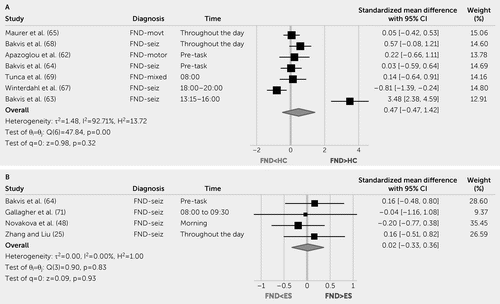
FIGURE 5. Forest plot of cortisol standardized mean difference between patients with functional neurological disorder (FND), epilepsy, and healthy control (HC) subjectsa
a Panel A shows a forest plot of baseline cortisol standardized mean difference between patients with FND and HCs. Panel B shows a forest plot of baseline cortisol standardized mean difference between FND and epilepsy patients. ES=epileptic seizures; θ=effect size; H2=heterogeneity; I2=variation; Q=homogeneity; REML=restricted maximum likelihood model; τ2=variability.
The results from five studies (four cohorts) comparing baseline cortisol levels in patients with FND-seiz compared with patients with epileptic seizures suggest no between-group differences (25, 48, 64, 70, 71) across morning (25, 48, 70, 71), evening (25, 48), and baseline levels prior to task performance (64). In the meta-analysis, the diagnosis of FND compared with epileptic seizures did not influence baseline cortisol levels (standardized mean difference=0.02, 95% CI=−0.33, 0.36; Figure 5B).
Task-related cortisol responses
Five studies compared cortisol levels in FND patients compared with HCs while performing a task (34, 35, 62–64). Across a variety of cognitive-affective tasks (i.e., Trier Social Stress test, approach avoidance, emotional Stroop task, awareness check, Stroop Color-Word test, or stressful mathematics), no group-by-time differences were observed (34, 35, 62, 63). However, one study reported that total salivary cortisol correlated with the adverse life events among patients with FND-movt (62). Within-group analyses found that salivary cortisol levels positively correlated with attentional bias for angry faces among patients with FND-seiz (64). In a postural control study, task-related salivary cortisol and movement parameters did not correlate (72).
Peri-ictal cortisol
Two studies compared peri-ictal cortisol levels (25, 73), with one demonstrating that the FND-seiz group had increased preictal serum cortisol levels compared with the epileptic seizures group (25). Separately, another study found that postictal plasma cortisol levels did not differentiate FND-seiz compared with epileptic seizures (73).
Adrenocorticotropic hormone (ACTH)
Baseline ACTH was measured in three cohorts across five studies (25, 66, 67, 70, 71). Of the studies that compared measurements with epileptic seizures, one group of investigators found that at-rest patients with FND-seiz had significantly decreased plasma ACTH (70, 71), while another study suggested no between-group differences in serum samples (25). Furthermore, studies that compared serum ACTH at rest between FND-seiz patients, HCs, and trauma control subjects determined that from 6:00 p.m. to 8:00 p.m. patients with FND-seiz had differentially increased ACTH levels and that baseline ACTH predicted diagnosis (66, 67). One study reported that patients with FND-seiz had higher pre-event and lower post-event serum ACTH levels compared with patients with epileptic seizures (25).
Amylase
Three studies measured salivary amylase during task performance (35, 62, 72). One study found that during the Trier Social Stress test, patients with FND-movt had higher baseline amylase levels compared with HCs, without group-by-time differences across the task (62). Additionally, during a mathematics performance task, there were no group differences among patients with FND-seiz compared with HCs (35). Relatedly, amylase levels and stressful mental arithmetic or speech task performances did not correlate across FND-movt patients and HCs (72).
Other Endocrine Markers
Compared with HCs, patients with mixed FND had increased urinary epinephrine excretion in the morning and decreased excretion in the afternoon (74–76). Other studies have examined between-group differences in a range of other markers without compelling findings to date (i.e., neuropeptide Y, norepinephrine, amino-terminal propeptide C-type natriuretic peptide, ghrelin, nefastin-1, testosterone, progesterone, oxytocin, and estradiol) (66, 67, 74–76).
Summary of Endocrine Findings
While useful for the study of individual differences, to our knowledge there are no compelling data to date to suggest that cortisol and ACTH levels reliably differentiate FND. More studies are needed to investigate the utility of other endocrine markers (i.e., neuropeptide Y, estradiol, and testosterone).
Inflammation Findings
Brain-derived neurotrophic factor (BDNF)
Two studies found that patients with FND had decreased serum BDNF compared with HCs (77, 78), observed in a mixed FND cohort (N=15) (77) and in patients with FND-seiz (N=12) (78). Neither study revealed FND-related differences when compared with patients with major depression (77) or epileptic seizures (78).
Other inflammation markers
In 79 children with mixed FND, researchers found that baseline C-reactive protein titers were above the normative range in 36 individuals (79). Group-level baseline differences showed that patients with mixed FND have increased platelets, immunoglobulin A, and immunoglobulin M (80, 81), as well as decreased lymphocyte count (81), compared with HCs; no group differences were observed for immunoglobulin G, TNF-alpha, IL-beta, or IL-6 levels (80, 82). Compared with patients with epileptic seizures peri-ictally, patients with FND-seiz have decreased white blood cell counts (83), serum creatine kinase (84–86), lactate dehydrogenase, and lactate but higher bicarbonate and anion gap levels (86–88).
Summary of inflammation findings
Initial data suggest that serum BDNF levels may differentiate patients with FND from HCs but do not reliably differentiate FND patients from neurological or psychiatric control subjects. Overall, potential inflammation markers in FND require more research.
DISCUSSION
This systematic review and meta-analysis indicate that pediatric patients with FND compared with HCs have increased resting HR and lower parasympathetic tone (rMSSD levels). Autonomic studies in adults with FND yielded mixed results; initial evidence showed increased baseline HR compared with HCs, decreased peri-ictal HR versus epileptic seizures, and differentiating HR patterns on tilt table testing for functional syncope. Although other autonomic and endocrine measurements did not reliably differentiate patients with FND from control subjects, individual differences in these indices related to clinically meaningful variables. For example, autonomic measurements related to illness duration (31), symptom severity (31), perceived stress (35), and mood or anxiety scores (53, 54, 60) in patients with FND. In terms of inflammation data, this literature is in its early stages.
Evidence suggests that children and adolescents with FND have increased autonomic arousal, a finding that advances our pathophysiological understanding of FND in this subgroup (89). Compared with HCs, pediatric FND showed increased HR and decreased HRV (rMSSD) at baseline and during cognitive and emotional tasks (26–28, 41). This suggests that compared with the prominent heterogeneity in adults with FND, autonomic markers reflecting increased sympathetic and decreased parasympathetic tone are more consistently present in this subpopulation. In one of the few studies analyzing neural circuit and autonomic data concurrently, Kozlowska et al. (28) showed that heighted arousal (indexed using HR values) served as a moderator of increased delta power in salience and default mode networks in patients with pediatric FND compared with HCs (28). Nonetheless, analyses also identified that autonomic activation patterns varied by age, attachment patterns, and clinical phenotype in children and adolescents with FND (26). These differences underscore the presence of heterogeneity even in pediatric FND, pointing out that there are likely multiple mechanistic pathways implicated in this population (89). Moving forward, it will be important to incorporate the diathesis-stress model of FND with developmental trajectories through the use of longitudinal studies (90). In the pediatric literature, more work is needed to directly compare children and adolescents with FND to neuropsychiatric control subjects, including individuals with chronic pain, anxiety, and personality disorders, to evaluate the specificity of these promising results.
The systematic review and meta-analysis findings also indicate that peri-ictal HR levels differentiated patients with FND-seiz from those with epileptic seizures. Specifically, patients with epileptic seizures showed increased HR during the ictal and postictal periods. Other autonomic markers such as SCA and SCR yielded similar results; however, more studies are needed to determine the effect of FND-seiz on these physiological parameters. Nevertheless, the already identified autonomic peri-ictal differences between FND-seiz and epileptic seizures are noteworthy, particularly given the multiyear delay between symptom onset and diagnosis in patients with FND-seiz. While the capture of typical events on video-electroencephalogram remains the gold standard for diagnosis (5), newly developed technologies can simultaneously measure HR, HRV, and SCA data, offering the opportunity to study the utility of composite diagnostic biomarkers in ambulatory and inpatient settings. Such an approach could help reduce the long diagnostic delays that are unfortunately far too common in this population. This wearable technology could also be extended beyond the spectrum of FND-seiz to aid the real-world capture of autonomic data across the full range of patients with FND.
The lack of high-quality studies investigating inflammation profiles in FND is an important gap in the literature, noteworthy given growing evidence showing a role for low-grade inflammation in the biology of a range of neuropsychiatric symptoms. For example, in a study investigating the biological and neurocognitive effects of a transient inflammatory response (i.e., typhoid vaccination) in heathy participants, self-reported fatigue correlated with activation changes in the posterior/mid insula and anterior cingulate cortex (20). In a separate study using the same paradigm, inflammation-associated mood changes correlated with decreased cingulate gyrus-amygdala connectivity (19). Furthermore, the combined influences of low-grade inflammation and perceived stress increased the risk of developing posttraumatic stress disorder (PTSD) in traumatized participants through disruptions in salience, default mode, and central executive network connectivity (91). Given that the onset of FND can be precipitated by physical injury, surgeries, or infections (1, 5) (processes that themselves are associated with inflammatory states), it will be impactful to further investigate possible associations between inflammation, brain networks, symptom severity, and prognosis across FND populations.
The heterogeneous findings identified across autonomic, endocrine, and inflammation data described in this article underscore the mechanistic, etiological, and methodological challenges of pathophysiology research in FND. To help illustrate the mechanistic complexity, an example is the role of the amygdala in this condition. Some studies have identified amygdala hyper-reactivity to affectively valenced stimuli, with time course data indicating impaired habituation (9) and heightened sensitization (8). However, using similar paradigms, others have reported no group-level differences or amygdala hypo-activations in patients with FND compared with HCs (35, 92). Notably, the amygdala has afferent connections to the hypothalamus, dorsal motor nucleus of the vagus, and periaqueductal gray that drive sympathetic and parasympathetic tone and stress-related hormonal responses. What remains unclear in patients with FND is whether amygdala hyperactivity is coupled with parallel heightened autonomic and endocrine (i.e., cortisol, amylase) profiles, or if there is a mismatch between neurocircuit profiles and downstream responses. This knowledge gap is important because it is possible that heightened amygdala activity could reflect a relative inefficiency in appropriately activating fight-or-flight and other defense response pathways. In support of the latter possibility is evidence that illness duration is associated with reduced integrity of amygdala-based white matter pathways (17, 18). Conversely, patients with FND have also demonstrated increased information flow (link-step connectivity) between the laterobasal (sensory) amygdala and the periaqueductal gray, suggesting that a limbic fast track in patients with FND may prime the nervous system for enhanced sympathetic responses (11).
Mechanistically, another concern in FND research is whether disease-related neurobiological processes are shared or distinct across FND subpopulations (e.g., FND-seiz compared with FND-movt). In support of a transdiagnostic approach are clinical observations that many patients experience mixed symptoms, and yet others presenting with one phenotype develop distinct functional neurological symptoms. Notably, imaging profiles correlated to symptom severity in a mixed FND cohort remained significant after adjusting for subtypes (11). Nonetheless, these questions remain actively debated, with some suggesting that FND-seiz should be considered a distinct entity from FND-movt (93).
Etiological heterogeneity likely also contributes to heterogeneous findings described across autonomic and endocrine data. For instance, stressful life events and early life maltreatment are well-known predisposing vulnerabilities for FND (94), with studies identifying that hypercortisolism was associated with trauma burden (62, 68). However, antecedent adverse life experiences were not systematically accounted for across studies in this review, and some patients with FND do not report a history of traumatic life events; differences in trauma burden across FND samples could be a major factor contributing to inconsistently identified results. Such themes raise the question of a possible trauma-subtype of FND (95).
Regarding methodological challenges, the multiplicity of additional physical symptoms (e.g., pain, fatigue) and commonly co-occurring neurological (e.g., traumatic brain injury) and psychiatric conditions (major depression, PTSD) that are themselves associated with altered autonomic, endocrine, and inflammation profiles are additional important considerations. For example, pain is a highly prevalent symptom in many patients with FND that is associated with a poor prognosis (4); stratifying FND patients by the presence or absence of prominent pain would likely help disentangle the heterogeneity present in the literature (96). Notably, pain is associated with heightened autonomic activity, increased inflammation, and endocrine alterations in other neuropsychiatric conditions (97). Similarly, PTSD is associated with hypercortisolism and elevated inflammation markers (98). To adequately account for these factors and other confounds (e.g., medication effects, time and type of cortisol sample drawn), larger samples are undoubtedly needed. Larger patient samples would also assist in comprehensively investigating the complex interplay between functional neurological symptoms and behavioral, psychological, autonomic, endocrine, inflammation, neuroimaging, and epigenetic and genetic data (99). Furthermore, replication of the same methods across studies need to be encouraged to allow for straight forward comparisons of results. More research is also needed to determine to what extent FND is mechanistically (and etiologically) similar or distinct across phenotypes. Lastly, given that several negative physiology studies found differences in self-report measures, as well as in cognitive and behavioral task performances, it is worth considering the overall utility (and framing) of biomarker research in this field.
In summary, the study of autonomic, endocrine, and inflammatory measurements in patients with FND remains a promising, yet complex, area of research. Given that FND is likely mechanistically and etiologically heterogenous, the study of biologically informed subtypes and composite biomarkers may be particularly fruitful future directions.
1 : A review and expert opinion on the neuropsychiatric assessment of motor functional neurological disorders. J Neuropsychiatry Clin Neurosci 2021; 33:14–26Link, Google Scholar
2 : Who is referred to neurology clinics? The diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010; 112:747–751Crossref, Medline, Google Scholar
3 : Assessment of emergency department and inpatient use and costs in adult and pediatric functional neurological disorders. JAMA Neurol 2021; 78:88–101Crossref, Medline, Google Scholar
4 : The prognosis of functional limb weakness: a 14-year case-control study. Brain 2019; 142:2137–2148Crossref, Medline, Google Scholar
5 : Evidence-based practice for the clinical assessment of psychogenic nonepileptic seizures: a report from the American Neuropsychiatric Association Committee on Research. J Neuropsychiatry Clin Neurosci 2021; 33:27–42Link, Google Scholar
6 : Structural alterations in functional neurological disorder and related conditions: a software and hardware problem? Neuroimage Clin 2019; 22:101798Crossref, Medline, Google Scholar
7 : Pathogenesis and pathophysiology of functional (psychogenic) movement disorders. Neurobiol Dis 2019; 127:32–44Crossref, Medline, Google Scholar
8 : Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One 2015; 10:e0123273Crossref, Medline, Google Scholar
9 : Emotional stimuli and motor conversion disorder. Brain 2010; 133:1526–1536Crossref, Medline, Google Scholar
10 : Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry 2014; 71:52–60Crossref, Medline, Google Scholar
11 : Corticolimbic fast-tracking: enhanced multimodal integration in functional neurological disorder. J Neurol Neurosurg Psychiatry 2019; 90:929–938Crossref, Medline, Google Scholar
12 : Impaired self-agency in functional movement disorders: a resting-state fMRI study. Neurology 2016; 87:564–570Crossref, Medline, Google Scholar
13 : The involuntary nature of conversion disorder. Neurology 2010; 74:223–228Crossref, Medline, Google Scholar
14 : Resting cortical PET metabolic changes in psychogenic non-epileptic seizures (PNES). J Neurol Neurosurg Psychiatry 2015; 86:1106–1112Crossref, Medline, Google Scholar
15 : Altered functional connectivity patterns of the insular subregions in psychogenic nonepileptic seizures. Brain Topogr 2015; 28:636–645Crossref, Medline, Google Scholar
16 : Facial emotion processing in patients with seizure disorders. Epilepsy Behav 2018; 79:193–204Crossref, Medline, Google Scholar
17 : Reduced limbic microstructural integrity in functional neurological disorder. Psychol Med 2021; 51:485–493Crossref, Medline, Google Scholar
18 : Microstructural integrity of affective neurocircuitry in patients with dissociative seizures is associated with emotional task performance, illness severity and trauma history. Seizure 2021; 84:91–98Crossref, Medline, Google Scholar
19 : Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 2009; 66:407–414Crossref, Medline, Google Scholar
20 : Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry 2009; 66:415–422Crossref, Medline, Google Scholar
21 : Biomarkers in functional movement disorders: a systematic review. J Neurol Neurosurg Psychiatry 2020; 91:1261–1269Crossref, Medline, Google Scholar
22 Study Quality Assessment Tools.
23 Movement-induced heart rate changes in epileptic and non-epileptic seizures. Arq Neuropsiquiatr 2009;67(3B):789–791Crossref, Medline, Google Scholar
24 : Comparison of heart rate variability parameters during complex partial seizures and psychogenic nonepileptic seizures. Epilepsia 2012; 53:1314–1321Crossref, Medline, Google Scholar
25 : Changes of serum adrenocorticotropic hormone and cortisol levels during sleep seizures. Neurosci Bull 2008; 24:84–88Crossref, Medline, Google Scholar
26 : Reduction of autonomic regulation in children and adolescents with conversion disorders. Psychosom Med 2015; 77:356–370Crossref, Medline, Google Scholar
27 : The respiratory control of carbon dioxide in children and adolescents referred for treatment of psychogenic non-epileptic seizures. Eur Child Adolesc Psychiatry 2017; 26:1207–1217Crossref, Medline, Google Scholar
28 : “Motoring in idle”: The default mode and somatomotor networks are overactive in children and adolescents with functional neurological symptoms. Neuroimage Clin 2018; 18:730–743Crossref, Medline, Google Scholar
29 : Impaired resting vagal tone in patients with functional movement disorders. Parkinsonism Relat Disord 2016; 30:18–22Crossref, Medline, Google Scholar
30 : Psychogenic non-epileptic seizures and functional motor symptoms: a common phenomenology? J Neurol Sci 2016; 368:49–54Crossref, Medline, Google Scholar
31 : Psychogenic non-epileptic seizures in children - psychophysiology & dissociative characteristics. Psychiatry Res 2020; 294:113544Crossref, Medline, Google Scholar
32 : Psychophysiological correlates of la belle indifférence. Arch Gen Psychiatry 1969; 20:239–245Crossref, Medline, Google Scholar
33 : Signs of autonomic arousal precede tilt-induced psychogenic nonsyncopal collapse among youth. Epilepsy Behav 2018; 86:166–172Crossref, Medline, Google Scholar
34 : Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia 2009; 50:1001–1011Crossref, Medline, Google Scholar
35 : fMRI response to acute psychological stress differentiates patients with psychogenic non-epileptic seizures from healthy controls: a biochemical and neuroimaging biomarker study. Neuroimage Clin 2019; 24:101967Crossref, Medline, Google Scholar
36 : Skin conductance response and emotional response in women with psychogenic non-epileptic seizures. Seizure 2020; 81:123–131Crossref, Medline, Google Scholar
37 : Ictal heart rate differentiates epileptic from non-epileptic seizures. Neurology 2002; 58:636–638Crossref, Medline, Google Scholar
38 : Pre- and postictal, not ictal, heart rate distinguishes complex partial and psychogenic nonepileptic seizures. Epilepsy Behav 2012; 23:68–70Crossref, Medline, Google Scholar
39 : Heart rate analysis differentiates dialeptic complex partial temporal lobe seizures from auras and non-epileptic seizures. Arq Neuropsiquiatr 2007; 65(3 A):565–568Crossref, Medline, Google Scholar
40 Autonomic nervous system functioning associated with psychogenic nonepileptic seizures: analysis of heart rate variability Epilepsy Behav 2016; 54:14–19Crossref, Medline, Google Scholar
41 : Activation of functional brain networks in children with psychogenic non-epileptic seizures. Front Hum Neurosci 2020; 14:339Crossref, Medline, Google Scholar
42 : Near-infrared spectroscopy in evaluating psychogenic pseudosyncope-a novel diagnostic approach. QJM 2020; 113:239–244Crossref, Medline, Google Scholar
43 : Heart rate variability measures as biomarkers in patients with psychogenic nonepileptic seizures: potential and limitations. Epilepsy Behav 2011; 22:685–691Crossref, Medline, Google Scholar
44 : Autonomic nervous system functions in interictal and postictal periods of nonepileptic psychogenic seizures and its comparison with epileptic seizures. Seizure 2010; 19:269–273Crossref, Medline, Google Scholar
45 : Heart rate variability parameters during psychogenic non-epileptic seizures: comparison between patients with pure PNES and comorbid epilepsy. Front Neurol 2020; 11:713Crossref, Medline, Google Scholar
46 : Emotional reactivity as a vulnerability for psychogenic nonepileptic seizures? Responses while reliving specific emotions. J Neuropsychiatry Clin Neurosci 2020; 32:95–100Link, Google Scholar
47 : Emotion in psychogenic nonepileptic seizures: responses to affective pictures. Epilepsy Behav 2012; 24:107–115Crossref, Medline, Google Scholar
48 : Diurnal patterns and relationships between physiological and self-reported stress in patients with epilepsy and psychogenic non-epileptic seizures. Epilepsy Behav 2017; 70(Pt A):204–211Crossref, Medline, Google Scholar
49 : Comparing maximum autonomic activity of psychogenic non-epileptic seizures and epileptic seizures using heart rate variability. Seizure 2016; 37:13–19Crossref, Medline, Google Scholar
50 : Electro-dermal arousal and self-appraisal in patients with somatization disorder. Indian J Clin Psychol 1998; 25:144–148Google Scholar
51 : Autonomic changes in psychogenic nonepileptic seizures: toward a potential diagnostic biomarker? Clin EEG Neurosci 2015; 46:16–25Crossref, Medline, Google Scholar
52 : The prolonged benzodiazepine withdrawal syndrome: anxiety or hysteria? Acta Psychiatr Scand 1990; 82:165–168Crossref, Medline, Google Scholar
53 : Explicit Facial Emotion Processing in Patients With Dissociative Seizures. Psychosom Med 2016; 78:874–885Crossref, Medline, Google Scholar
54 : Autonomic and subjective responsivity to emotional images in people with dissociative seizures. J Neuropsychol 2018; 12:341–355Crossref, Medline, Google Scholar
55 : Dissociation and interoception in functional neurological disorder. Cogn Neuropsychiatry 2020; 25:294–311Crossref, Medline, Google Scholar
56 : Anxiety in patients with hysterical conversion symptoms. J Neurol Neurosurg Psychiatry 1968; 31:490–495Crossref, Medline, Google Scholar
57 : Subjective and physiological response to emotions in temporal lobe epilepsy and psychogenic non-epileptic seizures. J Affect Disord 2019; 244:46–53Crossref, Medline, Google Scholar
58 : Organic and hysterical anesthesia; a method of differential diagnosis with the aid of the galvanic skin response. Am J Psychiatry 1945; 102:318–324Crossref, Medline, Google Scholar
59 : Startle responses in functional jerky movement disorders are increased but have a normal pattern. Parkinsonism Relat Disord 2017; 40:27–32Crossref, Medline, Google Scholar
60 : Clinical significance of the electrodermal habituation rate in anxiety disorders. Neuropsychobiology 1989; 22:68–71Crossref, Medline, Google Scholar
61 : Abnormal affective startle modulation in individuals with psychogenic [corrected] movement disorder. Mov Disord 2007; 22:1265–1271Crossref, Medline, Google Scholar
62 : Biological and perceived stress in motor functional neurological disorders. Psychoneuroendocrinology 2017; 85:142–150Crossref, Medline, Google Scholar
63 : Automatic avoidance tendencies in patients with psychogenic non-epileptic seizures. Seizure 2011; 20:628–634Crossref, Medline, Google Scholar
64 : Basal cortisol is positively correlated to threat vigilance in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2009; 16:558–560Crossref, Medline, Google Scholar
65 : A biological measure of stress levels in patients with functional movement disorders. Parkinsonism Relat Disord 2015; 21:1072–1075Crossref, Medline, Google Scholar
66 : Predicting psychogenic non-epileptic seizures from serum levels of neuropeptide Y and adrenocorticotropic hormone. Acta Neuropsychiatr 2019; 31:167–171Crossref, Medline, Google Scholar
67 : Vulnerability to psychogenic non-epileptic seizures is linked to low neuropeptide Y levels. Stress 2017; 20:589–597Crossref, Medline, Google Scholar
68 : Basal hypercortisolism and trauma in patients with psychogenic nonepileptic seizures. Epilepsia 2010; 51:752–759Crossref, Medline, Google Scholar
69 : Is conversion disorder biologically related with depression? A DST study. Biol Psychiatry 1996; 39:216–219Crossref, Medline, Google Scholar
70 : Endocrine abnormalities in human temporal lobe epilepsy. Yale J Biol Med 1987; 60:93–97Medline, Google Scholar
71 : Pituitary and adrenal function in epileptic patients. Epilepsia 1984; 25:683–689Crossref, Medline, Google Scholar
72 : Abnormal postural behavior in patients with functional movement disorders during exposure to stress. Psychoneuroendocrinology 2019; 101:232–239Crossref, Medline, Google Scholar
73 : Prolactin and cortisol levels in seizure disorders. J Assoc Physicians India 1994; 42:709–712Medline, Google Scholar
74 : Autonomic-humoral regulation in neuroses and neurotic development of the personality. Sov Neurol Psych 1983; 16:3–14Google Scholar
75 : Serum NT-pro CNP levels in epileptic seizure, psychogenic non-epileptic seizure, and healthy subjects. Neurol Sci 2018; 39:2135–2139Crossref, Medline, Google Scholar
76 : Time-dependent changes in the serum levels of prolactin, nesfatin-1 and ghrelin as a marker of epileptic attacks young male patients. Peptides 2011; 32:1276–1280Crossref, Medline, Google Scholar
77 : Serum brain-derived neurotrophic factor levels in conversion disorder: Comparative study with depression. Psychiatry Clin Neurosci 2007; 61:571–573Crossref, Medline, Google Scholar
78 : Decreased serum BDNF levels in patients with epileptic and psychogenic nonepileptic seizures. Neurology 2010; 75:1285–1291Crossref, Medline, Google Scholar
79 : Blood CRP levels are elevated in children and adolescents with functional neurological symptom disorder. Eur Child Adolesc Psychiatry 2019; 28:491–504Crossref, Medline, Google Scholar
80 : Serum immunoglobulin profiles of conversion disorder patients. Ger J Psychiatry 2008; 11:141–145Google Scholar
81 : Evaluation of mean platelet volume, red cell distributed width and neutrophil to lymphocyte ratio in conversion disorder. Neuropsychiatr Dis Treat 2019; 15:2879–2884Crossref, Medline, Google Scholar
82 : Proinflammatory cytokine levels in patients with conversion disorder. Acta Neuropsychiatr 2013; 25:137–143Crossref, Medline, Google Scholar
83 : Peripheral WBC count and serum prolactin level in various seizure types and nonepileptic events. Epilepsia 2001; 42:1472–1475Crossref, Medline, Google Scholar
84 : Diagnosis of psychogenic nonepileptic status epilepticus in the emergency setting. Neurology 2006; 66:1727–1729Crossref, Medline, Google Scholar
85 : Postictal serum creatine kinase in the diagnosis of seizure disorders. Arch Neurol 1985; 42:123–126Crossref, Medline, Google Scholar
86 : Muscle enzymes, blood gases and quantitative EEG in differential diagnosis of generalized tonic-clonic seizures and conversive non-epileptic seizures. Neurol Psychiatry Brain Res 2004; 11:133–136Google Scholar
87 : Lactate as a diagnostic marker in transient loss of consciousness. Seizure 2016; 40:71–75Crossref, Medline, Google Scholar
88 : The discriminative value of blood gas analysis parameters in the differential diagnosis of transient disorders of consciousness. J Neurol 2018; 265:2106–2113Crossref, Medline, Google Scholar
89 : A stress-system model for functional neurological symptoms. J Neurol Sci 2017; 383:151–152Crossref, Medline, Google Scholar
90 : Stress and functional neurological disorders: mechanistic insights. J Neurol Neurosurg Psychiatry 2019; 90:813–821Crossref, Medline, Google Scholar
91 : A double-hit of stress and low-grade inflammation on functional brain network mediates posttraumatic stress symptoms. Nat Commun 2020; 11:1898Crossref, Medline, Google Scholar
92 : Impaired emotion processing in functional (psychogenic) tremor: a functional magnetic resonance imaging study. Neuroimage Clin 2017; 17:179–187Crossref, Medline, Google Scholar
93 : Are psychogenic non-epileptic seizures just another symptom of conversion disorder? J Neurol Neurosurg Psychiatry 2017; 88:425–429Crossref, Medline, Google Scholar
94 : Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry 2018; 5:307–320Crossref, Medline, Google Scholar
95 : Early-life trauma endophenotypes and brain circuit-gene expression relationships in functional neurological (conversion) disorder. Mol Psychiatry (Epub ahead of print, Feb 12, 2020) Google Scholar
96 : Briquet syndrome revisited: implications for functional neurological disorder. Brain Commun 2020; 2:fcaa156 Crossref, Medline, Google Scholar
97 : Heart rate variability in patients with somatic symptom disorders and functional somatic syndromes: A systematic review and meta-analysis. Neurosci Biobehav Rev 2020; 112:336–344Crossref, Medline, Google Scholar
98 : Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015; 2:1002–1012Crossref, Medline, Google Scholar
99 : Effects of TPH2 gene variation and childhood trauma on the clinical and circuit-level phenotype of functional movement disorders. J Neurol Neurosurg Psychiatry 2020; 91:814–821Crossref, Medline, Google Scholar



