Role of White Matter Microstructure in Impulsive Behavior
Abstract
Objective:
Increased impulsivity is a hallmark trait of some neuropsychiatric illnesses, including addiction, traumatic brain injury, and externalizing disorders. The authors hypothesized that altered cerebral white matter microstructure may also underwrite normal individual variability in impulsive behaviors and tested this among healthy individuals.
Methods:
Impulsivity and diffusion tensor imaging (DTI) data were collected from 74 healthy adults (32 women; mean age=36.6 years [SD=13.6]). Impulsivity was evaluated using the Barratt Impulsiveness Scale–11, which provides a total score and scores for three subdomains: attentional, motor, and nonplanning impulsiveness. DTI was processed using the Enhancing Neuro Imaging Genetics Through Meta Analysis-DTI analysis pipeline to measure whole-brain and regional white matter fractional anisotropy (FA) values in 24 tracts.
Results:
Whole-brain total average FA was inversely correlated with motor impulsiveness (r=−0.32, p=0.007) and positively correlated with nonplanning impulsiveness (r=0.29, p=0.02); these correlations were significant after correction for multiple comparisons. Additional significant correlations were observed for motor impulsiveness and regional FA values for the corticospinal tract (r=−0.29, p=0.01) and for nonplanning impulsiveness and regional FA values for the superior fronto-occipital fasciculus (r=0.32, p=0.008).
Conclusions:
These results provide initial evidence that the motor and nonplanning subdomains of impulsive behavior are linked to specific white matter microstructural connectivity, supporting the notion that impulsivity is in part a network-based construct involving white matter microstructural integrity among otherwise healthy populations.
Excessive impulsivity is a hallmark finding among people with many psychiatric conditions, including substance use and hyperactivity and attentional disorders (1, 2). The specific neural circuits likely involve disrupted communications between frontal and subcortical and limbic brain structures, including the amygdala and nucleus accumbens, based on evidence ranging from the classical case of brain injuries in Phineas Gage to animal studies (3, 4). Neuroimaging studies of human subjects with, and at risk for, substance use disorders support this hypothesis. For example, increased impulsivity and reduced functional connectivity and microstructural integrity of white matter in cortico-striatal pathways predispose individuals to the development of impulsivity-driven phenotypes spanning many behaviors (5, 6). Here, we hypothesize that white matter integrity may be associated with normal between-subject variability in impulsivity measures. We tested this hypothesis with a large dataset of community-dwelling adults without psychiatric illness by mapping multiple domains of impulsivity using a self-reported questionnaire.
The Barratt Impulsiveness Scale–11 (BIS-11) is the most widely used assessment for impulsivity as a multidimensional behavior. The commonly used version consists of 30 questions that have been divided into three subdomains: attentional, motor, and nonplanning aspects of impulsiveness. The components of the BIS-11 have shown acceptable psychometric properties (7, 8), test-retest reliability (8), and clinical validity (9–11), raising an interesting question of whether some of these subcomponents can be mapped onto particular brain circuits. Therefore, a secondary aim here was to determine whether some of these impulsivity subdomains can be mapped onto specific white matter tracts.
Although it is an indirect measurement of white matter microstructure, fractional anisotropy (FA) from diffusion tensor imaging (DTI) has been extensively studied in its relationship to human behaviors, cognition, and health (12), thought to reflect, in part, the degree of myelination, although the exact biophysics remain complex and not fully delineated (13, 14). One previous study has examined the correlation between white matter and impulsivity among healthy community-dwelling individuals and found an interesting but seemingly paradoxical association in which higher FA in the prefrontal cortex-nucleus accumbens tract was associated with more impulsivity (15). However, it is unlikely that any one tract alone underserves such a multifaceted and complex behavior in its entirety. This study did not report results from other white matter tracts, which also left open the question of whether different white matter tracts in the brain should be systematically examined, not just in relationship to the overall impulsivity score but also to different subdomains of the impulsivity construct.
Our study attempted to more comprehensively approach the question of white matter contributions to impulsivity using BIS-11 total and subdomain scores. We then sought to rank the strength of the association between each impulsivity measure and the major white matter tracts to identify the white matter tracts that most strongly contribute to specific impulsivity measures.
Methods
This study was approved by the institutional review board at the University of Maryland, Baltimore. Each participant provided written informed consent for study participation.
Participants and Assessments
Healthy adults were recruited through local media advertisements in the Greater Baltimore area. Participants were evaluated using the Structured Clinical Interview for DSM-IV. Exclusion criteria were current psychiatric diagnosis, major medical or neurological illness, history of traumatic brain injury with cognitive sequelae, intellectual disability, substance dependence within the past 6 months, current substance abuse other than nicotine, and family history of psychosis in the prior two generations. A past single episode of depression was not an exclusion criterion if the adult was not currently experiencing depression.
BIS-11
All items of the BIS-11 are rated on a 4-point Likert scale, with a higher score indicating more impulsive behavior. Built into the questionnaire to avoid a response set were certain items worded to indicate nonimpulsiveness; these were accordingly reverse scored. The three subdomains were attentional impulsiveness, defined as being unable to focus on tasks at hand and intruding thoughts; motor impulsiveness, defined as acting on the spur of the moment and lack of lifestyle stability; and nonplanning impulsiveness, defined as enjoying mental challenges and planning or thinking deliberately. The total score and the three subdomain scores were the primary dependent variables.
DTI
All MR examinations were performed at the University of Maryland Center for Brain Imaging Research, using a Siemens 3T TRIO MRI (Erlangen, Germany) system equipped with a 32-channel phase array head coil. DTI data were collected using a single-shot, echo-planar, single refocusing spin-echo, T2-weighted sequence, with GeneRalized Autocalibrating Partially Parallel Acquisitions, acceleration factor 2 (16), yielding voxel dimensions 1.7 × 1.7 × 3.0 mm, acquisition time approximately 8 minutes. The sequence parameters were as follows: echo time-repetition time=87/8,000 ms, field of view=200 mm, axial slice orientation with 50 slices and no gaps, five b=0 images and 64 isotropically distributed diffusion-weighted directions with b=700 s/mm2. Enhancing Neuro Imaging Genetics Through Meta Analysis-DTI pipeline (https://www.nitrc.org/projects/enigma_dti) was used for tract-based spatial statistical analysis of diffusion anisotropy (17) with the protocol demonstrating excellent reproducibility (18, 19). FA images were created using previously published protocols (20, 21) from the Johns Hopkins University (JHU) Atlas of Human Functional Anatomy (22) and then nonlinearly aligned to a groupwise, minimal-deformation target brain using the FMRIB Linear Image Registration Tool method (17). In brief, the diffusion tensor was fit to the motion and eddy current diffusion data. RMSDIFF toolbox (23) was used to estimate the root mean square movement distance between diffusion-sensitized and b=0 images. The root mean square difference was calculated by comparing two 4 × 4 transformation matrixes: a transformation matrix from each frame to the first b=0 image and an identity matrix that served as the no-movement reference. The advantage of the root mean square distance includes both translation and rotation effects, providing an index of motion for the whole brain (24). Motion signal-to-noise ratio (SNR) was included in all covariate analysis (mean=1.1 [SD=0.59]). All data passed quality assurance control of 2.5-mm accumulated motion during the scan.
The quality assurance/quality control parameters were derived empirically as an adequate threshold in a test-retest examination of a subject for motion, as described previously by our group and others (18). The group’s minimal-deformation target brain was identified by warping all individual brain images in the group to each other. A group-average FA image was used to create a groupwise skeleton of 24 bilaterally averaged white matter tracts, thresholded at an FA level of 0.20 to eliminate non-white matter voxels, and FA values were projected onto the groupwise skeleton to account for residual misalignment among individual white matter tracts.
FA values were assigned to each point along a skeleton using the peak value found within a designated range perpendicular to the skeleton. This processing was performed under two constraints. A distance map was used to establish search borders for individual tracts. The borders were created by equally dividing the distance between two nearby tracts. Then, a multiplicative 20-mm full-width at half-maximum Gaussian weighting was applied during the search to limit maximum projection distance from the skeleton. Whole-brain averaged FA was obtained to first assess whether impulsivity measures were related to whole-brain white matter property in general. The JHU Atlas was used to separate white matter into the following tracts with average FA values along the spatial course: the genu, body, and splenium of corpus callosum, respectively; fornix (Fx; column and body); Fx-stria terminalis (FxST); internal capsule; anterior and posterior limb and retrolenticular part of the internal capsule, respectively; external capsule; corticospinal tract (CST); corona radiata (CR); anterior, posterior, and superior CR, respectively; posterior thalamic radiation; superior longitudinal fasciculus; inferior fronto-occipital fasciculus; superior fronto-occipital fasciculus (SFOF); cingulate gyrus; cingulum; uncinate fasciculus; and sagittal striatum—a total of 24 tracts. Because we had no a priori hypothesis with regard to hemispheric lateralization, we averaged the FA values for the individual left and right tracts, as in previous studies (20).
Data Analysis
Associations between FA values of the white matter and BIS-11 measures were calculated using partial correlations by covarying motion (SNR), age, and sex as well as cigarette smoking status effects because smoking has been associated with impulsivity (11) and white matter changes (25). All reported correlation coefficient values in this report were partial r values. For whole-brain average FA, a correlation was considered significant after false discovery rate (FDR) correction for multiple comparisons with a q-value set at <0.05. We also explored how each impulsiveness measure may be mapped to specific white matter tracts; the significance threshold was based on FDR with a q-value <0.05.
Results
Study Population
The study sample included 74 healthy adults (32 women, 42 men; mean age=36.6 years [SD=13.6], median age=32.9 years, range=19.1–62.4 years). Seven participants (9.5%) had a past single episode of depression. Eight (10.8%) had pharmacologically treated hypertension, none had a diagnosis of diabetes mellitus (type 1 or 2), and one (1.4%) had hypercholesterolemia. One participant (1.4%) had a remote history of breast cancer treated surgically, and none (0%) had any active treatment with chemotherapy or immunosuppressant medication.
Impulsivity and Whole-Brain White Matter
Whole-brain averaged FA was significantly associated with the motor impulsiveness component (r=−0.32, p=0.008) and nonplanning impulsiveness (r=0.30, p=0.015), both significant after FDR correction (q<0.05), and their correlations were in opposite directions (Figure 1). Whole-brain averaged FA was not significantly associated with the total impulsiveness score (r=−0.10, p=0.41) or the attentional impulsivity score (r=−0.14, p=0.26).
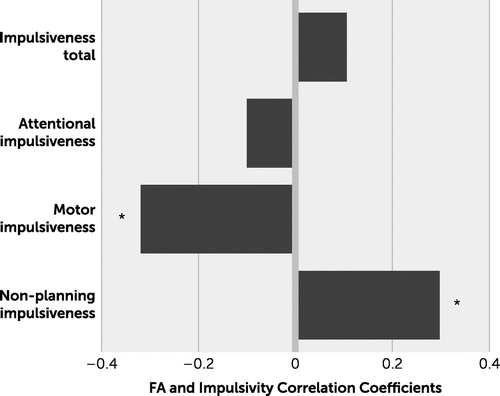
FIGURE 1. Correlation coefficients between impulsivity measures and whole-brain white matter fractional anisotropy (FA)
* Significance after false-discovery rate correction for multiple comparisons (q<0.05).
Mapping Impulsivity Onto White Matter Tracts
Exploratory analysis of total and subdomain impulsivity scores associated with individual white matter tracts revealed no significant associations after FDR correction for multiple comparisons (Figure 2A–2D). There were several nominally significant findings (p<0.05; p<0.01), including total impulsivity score and the CST (r=−0.24, p=0.05) (Figure 2A); attentional impulsiveness and the FxST (r=−0.29, p=0.018) and CST (r=−0.28, p=0.019) (Figure 2B); motor impulsiveness and the CST (r=−0.29, p=0.017), splenium of corpus callosum (r=−0.26, p=0.03), Fx (r=−0.26, p=0.03), and CR (r=−0.25, p=0.04) (Figure 2C); and nonplanning impulsiveness and the SFOF (r=0.32, p=0.008) as well as several others (Figure 2D). The white matter tracts showing the strongest association with motor impulsiveness (CST) and nonplanning impulsiveness (SFOF) were plotted in Figure 3, again showing that the correlations were in opposite directions for these two impulsivity measures.
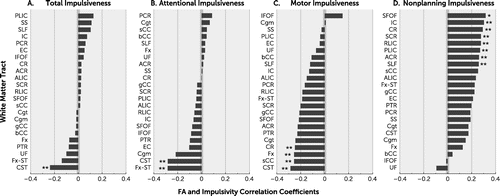
FIGURE 2. Associations of impulsivity measures and fractional anisotropy (FA) of 24 individual white matter tractsa
a Correlations with (panel A) total impulsiveness score and (panel B) attentional, (panel C) motor, and (panel D) nonplanning impulsiveness are shown. ACR=anterior corona radiata; ALIC=anterior limb internal capsule; bCC=body corpus callosum; Cgm=cingulum; Cgt=cingulate; CR=corona radiata; CST=corticospinal tract; EC= external capsule; Fx=fornix; FxST=fornix-stria terminalis; gCC=genu corpus callosum; IC=internal capsule; IFOC=inferior fronto-occipital fasciulus; PLIC=posterior limb internal capsule; PCR=posterior corona radiata; PTR=posterior thalamic radiation; RLIC=retrolenticular limb internal capsule; SCR=superior corona radiata; SFOF=superior fronto-occipital fasciculus; SLF=superior longitudinal fasciculus; sCC=splenium corpus callosum; SS=sagittal striatum; UF=uncinate fasciulus.
* p<0.01, **p<0.05.
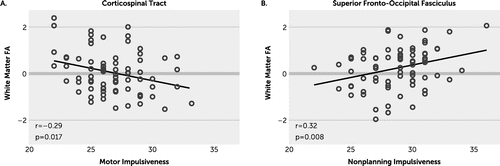
FIGURE 3. Plots of the tracts showing the strongest correlations with impulsivity subdomain scoresa
a Motor impulsiveness (panel A) is inversely correlated with the corticospinal tract white matter fractional anisotropy (FA), and (panel B) nonplanning impulsiveness is positively correlated with superior fronto-occipital fasciculus FA.
Associations With Demographic Characteristics
Age was not significantly associated with BIS-11 impulsivity total score (r=−0.15, p=0.22) and the motor and nonplanning subdomain scores (r=−0.10, p=0.42, and r=−0.01, p=0.90), respectively, but it was significantly associated with attentional impulsivity (r=−0.34, p=0.003). Sex was not significantly associated with BIS-11 impulsivity total and the three subdomain scores, F=0.15–2.1, df=1, 73, p=0.10–0.70). Current smoking status (coded as binary yes-no) was also not significantly associated with BIS-11 impulsivity total and the three subdomain scores, F=0.01–1.1, df=1, 73, p=0.3–0.9).
Discussion
Impulsivity is a complex behavior that has remained a challenge to study and understand operationally and neurobiologically. Barratt and colleagues (7) attempted to define this construct with the BIS-11 questionnaire. Despite its widespread use in studies of normal behaviors, psychopathology, and neuropathology (11, 26–28), recent debates have challenged its validity and interpretability (29, 30). However, genetic association studies using the BIS-11 have found evidence linking impulsiveness to serotonergic promoter region polymorphism (31) and motor impulsiveness to monoamine oxidase A genotype (32), providing validation support. With new technology to investigate the brain and its functions in vivo, we attempted to further map impulsiveness and the subdomains of this behavior onto structural connectivity in the brain of otherwise healthy adults.
The directions of the relationship between whole-brain FA and the motor component versus the nonplanning component of impulsivity are interestingly opposite. These findings may support recent attempts to redefine the psychometrics of the BIS-11 and its factor structure. For example, in a recent report analyzing the psychometric properties of the BIS-11 using a large community sample, the BIS-11 was most consistent with a two-factor model (29) rather than the traditional multidimensional representation. The proposed two factors, inability to wait for a reward and rapid response style, can be conceptually viewed as opposite: the former can be detrimental but the latter beneficial in everyday functions. Because motor impulsivity may be aligned with inability to wait for a reward and nonplanning impulsiveness may in part be related to rapid response style, a negative association between white matter FA for motor impulsivity and a positive association between white matter FA for nonplanning impulsivity may suggest that a poorer microstructural integrity of the whole-brain white matter is associated with motor impulsivity, but a higher integrity of the whole-brain white matter is associated with nonplanning impulsivity.
Higher FA in the white matter, including FA at the CR, anterior CR, and corpus callosum, has consistently been associated with faster information processing speed (33, 34). Because higher levels of nonplanning impulsiveness may reflect the rapid response style described in the two-factor model for BIS-11 (29), the finding that higher values of the CR FA are associated with higher scores on nonplanning impulsiveness may be consistent with the information processing speed function of the white matter. Moreover, nonplanning impulsivity has been found to be positively correlated with frontal gray matter volume, whereas motor and attentional subdomains were negatively correlated with superior temporal gray matter volume (35), in parallel with the signs of our findings of an association with white matter.
We observed an inverse association between the CST FA and motor impulsivity, suggesting that reduced FA here may contribute to more motor impulsivity. The CST underserves motoric functioning as its predominant responsibility, based on established neurobiological understandings; however, it has been shown to be more widely serving in psychological contexts as well (36). These findings support the notion that white matter studies may assist in understanding the underlying neurobiology of complex behavioral constructs such as impulsivity.
On other specific white matter tracts, we found no significant associations with impulsivity measures that survived corrections for multiple comparisons, although this may be due to insufficient power given the need to correct for the large number of tracts and the use of healthy controls in whom white matter integrity damage and psychopathology may be more subtle than in disease states. Nevertheless, we observed nominally significant associations that appear interpretable. For example, we found an association between lower FA at the CST and higher total impulsivity score (Figure 2A), consistent with impulsivity being largely a motor-based behavior, and impaired CST white matter microstructure may reduce impulse control output. Given that the CST was also found to trend significant in each of the other subdomains as well, these findings further strengthen our observations overall.
The white matter tract and impulsivity subdomain finding with the most association was between the SFOF and nonplanning impulsiveness. Given that nonplanning impulsiveness did reach significant correlation with whole-brain white matter FA, the SFOF is of interest. Functionally, this bundle is tasked with the role of modulating visual and spatial aspects of cognitive processing in a top-down fashion. The SFOF is responsible for processing information regarding the peripheral visual field, including aspects perceived outside of conscious cognitive processing, as discerned from lesion-based case studies, and controlling actions based on decisions with this input. Schmahmann and Pandya (37) proposed that the connectivity between dorsolateral prefrontal and parieto-occipital areas served by this fronto-occipital fasciculus are key in the higher order aspects of motor behavior and visuospatial aspects of attention processes. Our findings of nonplanning impulsiveness mapping onto the SFOF are in stark agreement with the behavioral implications reported elsewhere.
Few robust studies of DTI and impulsivity exist. A report of 10 chronic marijuana users compared with nonmarijuana users found an association between BIS-11 score and white matter FA but only among marijuana users (38). Another study used the Urgency, Premeditation, Perseverance, and Sensation Seeking questionnaire to measure impulsivity among 143 healthy control subjects (15) and found that the FA of the white matter tract between prefrontal cortex and nucleus accumbens was positively correlated with impulsivity levels. The direction of the association may be counterintuitive, but these findings also support a role of white matter integrity in impulsivity that bears relevance in healthy control subjects.
Impulsivity among humans is unlikely to be controlled by isolated neuroanatomy or neurotransmitter domains. It is likely a rather complex multifactorial human behavior, influenced by multiple cortical and subcortical brain regions, in which connectivity by cerebral white matter may serve a critical role. Regional laterality may also have importance with regard to white matter microstructure and is a key difference when compared with functional neuroanatomy, in which lateralized brain regions are ubiquitously assumed to confer distinct outputs. In agreement with the largest and most robustly used DTI analytic method to date, an agnostic approach to laterality was used in the main analysis (18, 39, 40) here as well. With accumulating data, however, lateralized microstructural integrity may similarly prove useful. We have shown here that aspects of impulsive behavior could be attributed to the integrity of the white matter microstructure connecting brain regions implicated in impulsive behaviors.
The BIS-11 is a self-reported questionnaire and, as such, is subject to user biases, including lack of insight among the participants reporting. The cross-sectional nature of the study design also limits causal interpretation between white matter integrity and impulsivity measures. Diffusion MRI parameters other than FA (such as axial, radial, and mean diffusivities) were not explored, although FA has been used as a more accurate composite means of capturing microstructural integrity. The demographic characteristics were constrained in several important dimensions, including substance use disorder over the course of the individuals’ lifetime, and subjects were only screened for more recent (6-month timeframe) habitual use of substances. Data on factors that may also contribute to white matter microstructural impairments, such as pollution and pesticide exposure, were not collected, and these should be considered in future studies. Also, white matter macrostructure data that include hyperintensities would similarly add value to future studies assessing microstructural integrity. Last, tract-specific analysis was limited by insufficient power to correct for multiple comparisons, so the tract-based interpretations should be viewed with caution; however, the findings were generally in agreement with the averaged whole-brain FA findings.
Conclusions
Impulsivity is a common human characteristic that exists on a spectrum, and attempts have been made to better define the underlying neurobiology. Using DTI neuroimaging, we showed that the averaged whole-brain white matter FA is associated with motor and nonplanning subdomains of impulsivity, and evidence also suggests tract specificity in which CST is associated with total impulsivity; cingulate, cingulum, and CR are associated with motor impulsiveness; and CR is associated with nonplanning impulsiveness. These findings support future studies exploring causal relationships between white matter microstructural integrity and impulsive behavior, as well as investigation of these relationships in states of greater impulsivity, as in several neuropsychiatric diseases.
1 : Individual differences in impulsive action and dopamine transporter function in rat orbitofrontal cortex. Neuroscience 2016; 313:122–129Crossref, Medline, Google Scholar
2 : Decreased prefrontal cortical dopamine transmission in alcoholism. Am J Psychiatry 2014; 171:881–888Crossref, Medline, Google Scholar
3 : Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 2007; 315:1267–1270Crossref, Medline, Google Scholar
4 : Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci 2017; 18:158–171Crossref, Medline, Google Scholar
5 : Striatal activity and reduced white matter increase frontal activity in youths with family histories of alcohol and other substance-use disorders performing a go/no-go task. Brain Behav 2015; 5:e00352Crossref, Medline, Google Scholar
6 : Combining diffusion tensor imaging and magnetic resonance spectroscopy to study reduced frontal white matter integrity in youths with family histories of substance use disorders. Hum Brain Mapp 2014; 35:5877–5887Crossref, Medline, Google Scholar
7 : Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol 1995; 51:768–774Crossref, Medline, Google Scholar
8 : Fifty years of the Barratt Impulsiveness Scale: an update and review. Pers Individ Dif 2009; 47:385–395Crossref, Google Scholar
9 Impulsivity and time of day: effects on performance and cognitive tempo. Pers Indiv Differ. 1998;26:199-207.Crossref, Google Scholar
10 : Electrophysiological substrates of impulsiveness: potential effects on aggressive behavior. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29:305–313Crossref, Medline, Google Scholar
11 : Impulsivity in smoking, nonsmoking, and ex-smoking alcoholics. Addict Behav 2004; 29:973–978Crossref, Medline, Google Scholar
12 : Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging 2012; 33:9–20Crossref, Medline, Google Scholar
13 : Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003; 20:1714–1722Crossref, Medline, Google Scholar
14 : Fractional anisotropy of cerebral white matter and thickness of cortical gray matter across the lifespan. Neuroimage 2011; 58:41–49Crossref, Medline, Google Scholar
15 : White matter integrity in the fronto-striatal accumbofrontal tract predicts impulsivity. Brain Imaging Behav 2018; 12:1524–1528Crossref, Medline, Google Scholar
16 : Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002; 47:1202–1210Crossref, Medline, Google Scholar
17 : Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage 2013; 81:455–469Crossref, Medline, Google Scholar
18 : Reproducibility of tract-based white matter microstructural measures using the ENIGMA-DTI protocol. Brain Behav 2017; 7:e00615Crossref, Medline, Google Scholar
19 : Reproducibility of quantitative structural and physiological MRI measurements. Brain Behav 2017; 7:e00759Crossref, Medline, Google Scholar
20 : Cortisol reactivity to stress and its association with white matter integrity in adults with schizophrenia. Psychosom Med 2015; 77:733–742Crossref, Medline, Google Scholar
21 : Fornix structural connectivity and allostatic load: empirical evidence from schizophrenia patients and healthy controls. Psychosom Med 2017; 79:770–776Crossref, Medline, Google Scholar
22 : Fiber tract-based atlas of human white matter anatomy. Radiology 2004; 230:77–87Crossref, Medline, Google Scholar
23 : Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(Suppl 1):S208–S219Crossref, Medline, Google Scholar
24 : Retrospective motion correction protocol for high-resolution anatomical MRI. Hum Brain Mapp 2006; 27:957–962Crossref, Medline, Google Scholar
25 : Characterizing white matter changes in cigarette smokers via diffusion tensor imaging. Drug Alcohol Depend 2014; 145:134–142Crossref, Medline, Google Scholar
26 : Unique aspects of impulsive traits in substance use and overeating: specific contributions of common assessments of impulsivity. Am J Drug Alcohol Abuse 2014; 40:463–475Crossref, Medline, Google Scholar
27 : Manic symptoms and impulsivity during bipolar depressive episodes. Bipolar Disord 2007; 9:206–212Crossref, Medline, Google Scholar
28 : Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am J Psychiatry 2005; 162:1680–1687Crossref, Medline, Google Scholar
29 : The Barratt Impulsiveness Scale-11: reassessment of its structure in a community sample. Psychol Assess 2013; 25:631–642Crossref, Medline, Google Scholar
30 : A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions. Transl Psychiatry 2017; 7:e1096Crossref, Medline, Google Scholar
31 : A pilot genetic study of the continuum between compulsivity and impulsivity in females: the serotonin transporter promoter polymorphism. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29:713–717Crossref, Medline, Google Scholar
32 : Monoamine oxidase—a genetic variants and childhood abuse predict impulsiveness in borderline personality disorder. Clin Psychopharmacol Neurosci 2017; 15:343–351Crossref, Medline, Google Scholar
33 : Association of white matter with core cognitive deficits in patients with schizophrenia. JAMA Psychiatry 2017; 74:958–966Crossref, Medline, Google Scholar
34 : The common genetic influence over processing speed and white matter microstructure: evidence from the Old Order Amish and Human Connectome Projects. Neuroimage 2016; 125:189–197Crossref, Medline, Google Scholar
35 : Neural correlates of impulsivity factors in psychiatric patients and healthy volunteers: a voxel-based morphometry study. Brain Imaging Behav 2011; 5:52–64Crossref, Medline, Google Scholar
36 : Diffusion anisotropy of the internal capsule and the corona radiata in association with stroke and tumors as measured by diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2001; 22:456–463Medline, Google Scholar
37 : The complex history of the fronto-occipital fasciculus. J Hist Neurosci 2007; 16:362–377Crossref, Medline, Google Scholar
38 : Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp Clin Psychopharmacol 2011; 19:231–242Crossref, Medline, Google Scholar
39 : White matter microstructure and its relation to clinical features of obsessive-compulsive disorder: findings from the ENIGMA OCD Working Group. Transl Psychiatry 2021; 11:173Crossref, Medline, Google Scholar
40 : Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry 2018; 23:1261–1269Crossref, Medline, Google Scholar



