Psychotherapy for PTSD: Neuroimaging of Recovery Processes
Abstract
Advancement in research related to the neuroscience of psychotherapy for posttraumatic stress disorder (PTSD) is in the early stages. Although mechanisms of recovery have been postulated from existing psychological theories, relatively little is confirmed regarding actual changes that occur at the neurobiological level. Eventual goals include the ability to predict treatment outcome and to improve patient–treatment matching. This would be highly significant for veterans with PTSD, as dropout rates for treatment are high for this cohort (average, 23% in research studies).6 Residual symptoms often remain even after successful psychotherapy.6
Much of the neuroimaging research related to PTSD has focused on areas and processes believed to be important for fear learning and extinction (Figure 1). In brief, areas of ventromedial prefrontal cortex (PFC), particularly the anterior cingulate cortex, are thought to be involved in the extinction of conditioned fear.7 This region provides an inhibitory influence on the amygdala, an area essential to fear learning and expression and mediation of responses to fear and stress.8,9 The most studied structure is the hippocampus because of its importance for declarative memory.7,10 The hippocampus and surrounding regions are important in contextualization of one’s environments in response to threat, modulating fear responses based on personal context and extinction of conditioned fear.10 These regions operate together to accomplish adaptive fear conditioning, expression, and extinction in healthy individuals. However, learned fear is only one aspect of PTSD. More recent studies have begun to explore other areas and circuits, as well.
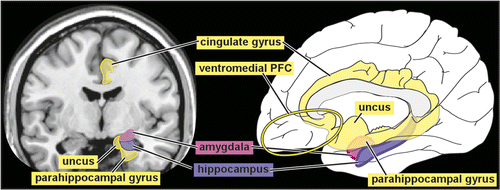
Neuroimaging of PTSD
A major challenge for research into the neurobiology of PTSD is the multiple sources of variability across patients. Although a diagnosis of PTSD requires presence of specific symptom clusters in response to a traumatic event (e.g., intrusion and avoidance symptoms, alterations in arousal/reactivity, negative changes in cognitions and mood), the clinical presentation is diverse. There is also considerable heterogeneity with regard to type and duration of trauma(s) experienced, age at traumatization, chronicity of symptoms, primacy of various emotions (e.g., fear, guilt, shame), and level of impairment. The neurobiology of recovery may also differ across individuals, as both compensatory processes and normalization of function may occur. Neuroimaging studies generally use small groups, so individual differences in subjects studied may greatly impact results. Meta-analytic techniques have been developed that allow data to be combined across studies. In addition, studies that focus on a more homogeneous set of subjects may provide more consistent results. Thus, findings from studies that used subjects relevant to combat-related PTSD will be emphasized.
The vast majority of neuroimaging research uses MRI scans for visualization of structure. Electrophysiological techniques such as electroencephalography and magnetoencephalograpy allow direct assessment of brain activity. Other functional imaging techniques provide indirect measures, most commonly based on cerebral blood flow (CBF). Single photon emission tomography (SPECT) and positron emission tomography (PET) provide relative or quantitative measures of regional CBF or metabolic rate. Functional MRI (fMRI) and near infrared spectroscopy both provide a relative measure of neuronal activity based on activation-induced increase in CBF and blood oxygenation.
Functional Neuroimaging
As noted above, much of functional imaging of PTSD has focused on the fear system. Hyperreactivity of the amygdala, insula, and other limbic areas and hypoactivity of the ventromedial PFC have been the most emphasized findings in reviews, with variable results noted for the hippocampus.7,11–13 Meta-analytic studies that combined data across types of tasks (e.g., cognitive control, symptom provocation, affective or fear processing) reported hyperactivation within and around the amygdala and areas of both hyper- and hypoactivation within the cingulate cortex, with variable findings in other areas.14–16 One meta-analysis found that separating studies by the control groups used (trauma exposed or trauma naïve) made a significant difference in the results.15 Hyperactivation of the hippocampus and amygdala was only found when subjects with PTSD were compared with trauma-naïve control subjects. In addition, the location of hyperactivation within the insula was more anterior. As noted by the authors of this study, these results suggest that hyperreactivity within limbic regions may be common in all trauma-exposed individuals and not just those who develop PTSD.15 Two recent meta-analyses have taken this refinement a step further. They limited the studies included by task (symptom provocation, cognitive-emotional) and control group (trauma exposed).1,2 Both reported hyperactivation in several areas within the cingulate cortex (dorsal anterior, middle, posterior) during symptom provocation, but findings diverged considerably for other parts of the cortex (Figure 2). Neither meta-analysis reported changes in the hippocampal region or in the insula. Hyperactivation of the amygdala was found only to cognitive-emotional tasks and only when region-of-interest studies were included.1 Thus, recent meta-analyses have confirmed the need for a more nuanced approach.

Some attempts have been made to refine methodology by studying similar patients, specific symptom clusters, and/or subtypes of PTSD. Even studies involving relatively homogeneous samples (e.g., combat veterans) do not yet appear convergent.17–22 However, a study of combat-related PTSD in identical twins suggests that increased activation of dorsal anterior cingulate regions (cognitive interference task) may be a familial risk factor for PTSD rather than acquired after trauma.23 The few studies that focused on particular symptom clusters have implicated multiple subcortical and cortical regions, as well as midbrain areas.18,24–26 Dividing PTSD into dissociative and hyperarousal subtypes may be a fruitful line of inquiry, as these subtype are thought to differ greatly in modulation of emotion (overmodulation versus undermodulation).27–29 Studies are beginning to explore major networks (e.g., default mode network, salience network), but this literature remains largely preliminary.30–33
Structural Neuroimaging
There is evidence to suggest that total brain volume is reduced in both traumatized children and adults compared with trauma-naïve individuals.34–36 A meta-analysis did not find a significant difference in brain volume when adults with PTSD were compared with trauma-exposed control subjects.34 However, a longitudinal study of PTSD patients found accelerated atrophy (rate greater than control group) in widespread areas of the brain in the PTSD subgroup with worsening symptoms.37 There was only a single small area of accelerated atrophy in the PTSD subgroup with stable or improving symptoms.37 Similarly, a study of police officers reported a positive correlation between number of traumatic exposures and ventricular volume (indirect measure of atrophy).38
A recent systematic review found that smaller total hippocampal volume is the most frequently reported structural difference in studies comparing individuals with PTSD to healthy control subjects.39 This review and an older meta-analysis both reported mostly right-sided reductions.39,40 Similarly, a recent study in combat veterans with chronic and current PTSD found that PTSD duration was inversely correlated with hippocampal volume, but only on the right.41 However, two meta-analyses that focused on whole brain studies that included trauma-exposed control subjects reported left laterality.42,43 Gray matter reductions were localized to the anterior region of the hippocampus. A recent study in combat veterans with PTSD also found reduced volume in the left but not right hippocampus compared with trauma-exposed veterans.44 Other studies, including some in combat veterans, have not found volume differences in this structure.11,37,39,45–47 A challenge in this area of research has been to distinguish whether a smaller hippocampus is a risk factor for PTSD or is acquired after trauma. The inverse correlation noted above between PTSD duration and hippocampal volume could indicate an effect of PTSD or could indicate a risk factor for development of persistent symptoms.41 Strong evidence from an identical twin study suggests low hippocampal volume is a risk factor for combat-related PTSD.39,48,49 Another study in combat veterans reported that current PTSD was associated with smaller total hippocampal volume, whereas remitted PTSD was not.50 A prospective longitudinal (18 months) study of active duty military personnel divided the group by direction of change in left hippocampal volume (manual segmentation).51 The combat paramedics who had volume decreases developed more severe PTSD symptoms, whereas those with volume increases did not. Notably, premilitary hippocampal volumes did not predict symptom development.51
There was considerable agreement between two recent meta-analyses of whole brain studies comparing gray matter density between individuals with PTSD and trauma-exposed control subjects.42,43 In addition to the reduced volume of left hippocampus reported above, both found reductions within adjacent areas of dorsal anterior cingulate and left rostral middle temporal cortices. They disagreed on reductions in the right dorsolateral PFC. One meta-analysis also included comparison to trauma-naïve control subjects.42 There was no overlap of regions with reduced gray matter density found between the two comparisons (PTSD versus trauma-exposed control subjects, PTSD versus trauma naïve control subjects).42 In contrast, a study using a different meta-analytic technique reported reduced gray matter in the dorsal anterior cingulate compared with both trauma-exposed and trauma-naïve control subjects.52 This study did not find any differences in the middle temporal cortex or hippocampus but did report reduced gray matter in the left anterior insula and right parahippocampal cortices when individuals with PTSD were compared with trauma-exposed control subjects. They also reported PTSD symptom severity [as measured with the Clinician Administered PTSD Scale (CAPS)] correlated with decreased gray matter in the left dorsal anterior cingulate and increased gray matter in the left posterior insular cortices.52 Several recent studies have assessed the relationship between CAPS and regional brain structure (e.g., gray matter volume and cortical thickness) in combat veterans.19,44,45,53–55 Both smaller and larger amygdala volumes have been reported to be associated with PTSD in this cohort.44,53 Two research groups have reported a negative correlation between CAPS and the middle temporal gyrus that was not found by a third group.19,45,54,55 Studies diverged on other brain areas (e.g., subgenual anterior cingulate cortex, insula, paracentral/posterior cingulate cortex, supplementary motor cortex, middle occipital cortex, caudate, hypothalamus). Factors identified as potentially underlying these differences across studies include presence of early life trauma, current symptom severity and/or symptom presentation, and presence/absence of mild traumatic brain injury.45,53–55 An earlier identical twin study comparing combat-exposed twins with and without PTSD to trauma-naïve twins found evidence for acquired volume reduction in the right hippocampus, dorsal/pregenual anterior cingulate, and insular cortices.56
Although a number of studies have correlated structural abnormalities with specific PTSD symptoms (e.g., re-experiencing, dissociation, hyperarousal), there are considerable differences in the findings. Two studies found smaller occipital cortex volume to be related to re-experiencing subscores, although the studies did not overlap otherwise.57,58 Studies of re-experiencing symptoms in combat veterans have implicated volume reductions in other areas (e.g., insula, anterior cingulate cortex, amygdala, thalamus).38,56 One study has reported a positive correlation between trait dissociation scores and volume within several regions of prefrontal cortex in both subjects with PTSD and trauma-exposed control subjects.59 Another study reported that hyperarousal symptoms were associated with smaller volume of right dorsal anterior cingulate cortex in subjects with child abuse–related PTSD compared with trauma-naïve control subjects.60 In contrast, hyperarousal symptoms were associated with smaller volume of the posterior left middle temporal gyrus in combat veterans.61 A smaller volume of subgenual anterior cingulate cortex and bilateral insula has been correlated with avoidance in combat veterans.45
Recovery From PTSD
Neuroimaging studies that explore neural correlates of psychotherapy-induced recovery may provide further insight to processes underlying PTSD. In the absence of meta-analyses, a focus on the most relevant individual studies may provide a basis for differentiating compensatory changes from neurobiological normalization. Notably, changes associated with symptom reduction are expected to be diverse due to the heterogeneous nature of PTSD.
Functional Neuroimaging
Two studies have used electrophysiological methods to directly assess brain activity evoked by symptom provocation prior to and following treatment. A study that assessed cortical activation (EEG) while viewing trauma-related pictures in subjects with PTSD compared with wait-list control subjects reported that, prior to treatment, both groups exhibited greater activation in the right hemisphere.62 Following successful treatment [cognitive behavioral therapy (CBT)], there was a reduction in right hemisphere activation in frontal regions. This correlated with symptom improvement, suggesting normalization.62 In the other EEG study, PTSD subjects were assessed before and after treatment while listening to a script of their trauma.63 Before treatment, the PTSD group exhibited a significantly higher cortical activation than trauma-exposed control subjects in the bilateral orbitofrontal and anterior cingulate cortices (delta, alpha, and beta 1 bands), bilateral parahippocampal gyri (delta and theta bands), and bilateral posterior cingulate cortex (theta band). After successful treatment (eye movement desensitization and reprocessing therapy), the PTSD group demonstrated significantly increased cortical activations during script listening in the right fusiform gyrus (delta, theta, alpha, and beta 1 bands) and the visual cortex (delta and theta bands). A connectivity analysis revealed decreased connectivity (theta band) between the left visual cortex and right fusiform gyrus. The authors of this study noted that these results suggest a shift in maximal cortical activation during script listening from prefrontal and limbic areas to primary and higher-order visual areas and normalization of visual processing.63 Thus, both EEG studies suggested that treatment promoted normalization of processing.
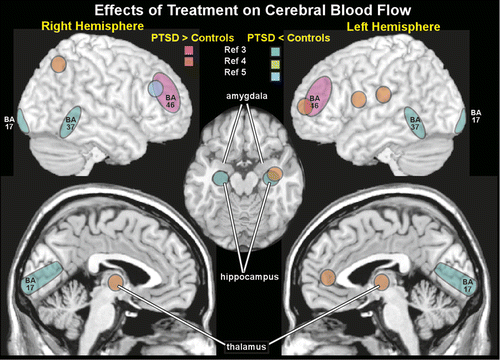
Several studies have used indirect functional imaging methods to assess treatment-related changes. Three studies have reported changes in CBF (SPECT). Two studies compared CBF increases induced by listening to a personal trauma script in subjects with PTSD to both waitlist and trauma-exposed control subjects.3,5 In one study, CBF was higher in the right insula and superior/middle frontal gyri in the PTSD group compared with trauma-exposed control subjects.5 Treatment (brief eclectic psychotherapy) was associated with decreased CBF in the right middle frontal gyrus, suggesting some normalization of function (Figure 3). CBF was decreased in the right uncus in waitlist control subjects. Symptom improvement correlated with CBF changes in the bilateral superior/middle frontal gyrus and left superior temporal gyrus. In the other study, CBF was decreased in bilateral temporal poles, mesial temporal, superior/middle temporal, and orbitofrontal cortices compared with trauma-exposed control subjects.3 Following treatment (eye movement desensitization and reprocessing therapy), pretreatment differences (volume of interest) in CBF between treatment responders (11 of 15 patients) and trauma-exposed control subjects were no longer significant, suggesting normalization. However, within-subject analyses did not reveal significant changes pre-post treatment. When treatment responders (11/15) were compared with nonresponders (4/15), CBF was decreased in the temporal cortex (BA37), visual cortex (BA17), and hippocampus; CBF was increased in the dorsolateral PFC (BA46) (Figure 3). The third study examined CBF changes in subjects with subthreshold PTSD before and after treatment and waitlist control subjects during recall of trauma-related material.4 After successful therapy (brief psychotherapy with cognitive restructuring and exposure components), within-subject analyses identified significantly decreased CBF in the left amygdala and increased CBF in the left dorsal anterior cingulate cortex, dorsolateral and ventrolateral PFC, and hippocampus, as well as bilateral parietal lobes (BA 40, BA 7) and thalamus (Figure 3). Correlational analyses indicated significant positive correlations between the change in CBF of the left PFC and both the left thalamus and left parietal lobe. Furthermore, symptom reduction was positively correlated with the left PFC CBF and negatively correlated with the amygdala CBF.4 Another study used near infrared spectroscopy to measure functional activation (based on increased regional concentration of oxyhemoglobin) during trauma recall within the lateral prefrontal cortex in subjects with PTSD prior to and following eye movement desensitization and reprocessing therapy.64 After treatment, within-subject analyses indicated significantly less activation in this area; these changes were associated with reduction in symptoms. An fMRI study compared brain activations during trauma reminders in police officers with subthreshold PTSD to both waitlist and trauma-exposed control subjects (all police officers).65 At baseline, both subthreshold PTSD groups had higher activity in the left amygdala and lower activity in the orbitofrontal and medial PFC than trauma-exposed control subjects. Following treatment (cognitive restructuring and exposure therapy), activity was increased in the orbitofrontal and medial PFC and decreased in the amygdala, suggesting normalization of activity. Another fMRI study compared brain activations during an emotional face memory task early in treatment for PTSD and 6–9 months later, when 65% no longer met diagnostic criteria.66 There was a positive correlation between memory-related activation in the amygdala (modulation of memory by emotion) and ventromedial PFC (episodic memory) and CAPS at both time points. In contrast, changes in memory-related activation in the anterior hippocampus and subgenual anterior cingulate cortex significantly correlated with degree of recovery, but not with current CAPS. Studies using other types of tasks (e.g., attention, concentration, social cognition, emotional faces, affective Stroop) have also documented changes in brain activations following treatment.67–72 Although differing greatly in both research methodology and subject characteristics, overall, these studies indicate that effective psychotherapy can be associated with both compensatory changes and normalization of brain activity.
Structural Neuroimaging
There is limited evidence for structural changes associated with recovery from PTSD as a result of psychotherapy. As noted previously, a study comparing veterans with current PTSD or remitted PTSD to both trauma-exposed and trauma-naïve veteran control subjects reported that there were no significant differences in volume between the remitted PTSD group and either control group.50 A study of police officers with PTSD reported no increase in hippocampal volume (manual segmentation) after successful psychotherapy in comparison to trauma-exposed control subjects, although the PTSD group had smaller volumes before treatment.73 It is important to note that half of the PTSD group had a history of previous psychotherapy for trauma-related symptoms and therefore may have been somewhat treatment resistant. Another study did find volumetric changes in the hippocampus (manual segmentation) after multiple sessions of eye movement desensitization and reprocessing therapy.74 Within-subject analyses demonstrated increased volume bilaterally, but the study had some methodological weaknesses including lack of a control group. Another larger and more methodologically rigorous study comparing subjects with PTSD to trauma-exposed control subjects reported that differences in hippocampal volume (automated segmentation, smaller in PTSD) were no longer present after trauma-focused CBT.75 Within-subject comparisons offered stronger evidence for this change and confirmed greater hippocampal volumes in PTSD patients after psychotherapy. Notably, symptom reduction was correlated with changes in hippocampal volume.
Conclusions
Although there is much still to be learned, the evidence to date indicates that both compensatory processes and normalization of function may occur during recovery from PTSD. As discussed above, PTSD is a heterogeneous condition. Many investigators are moving toward a more nuanced approach, narrowing samples by factors such as type of trauma, chronicity of symptom, predominant symptom cluster, and age of PTSD onset. The choice of control group is also of great importance, as trauma exposure alone has been shown to affect brain structure and function. The increasing use of whole-brain analytic approaches is broadening investigations beyond particular regions of interest that have been historically subject to heavy focus. Studies that capture functional changes in networks rather than single areas may also provide valuable insights. The need to address many methodological concerns, such as test–retest reliability of neuroimaging methods and standardizing procedures to promote replication, has also been noted.76 Neuroscience of psychotherapy is challenging, as each method of assessing brain structure, function, and connectivity possesses strengths and weaknesses. Spatial and time resolution vary between methods and are constantly improving, making even the most recent meta-analyses slightly out of date. Nevertheless, such research provides a foundation for understanding how recovery from PTSD occurs at the neurobiological level and may also provide insight into the impact of various forms of psychotherapy.
1 : Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord 2012; 2:9Crossref, Medline, Google Scholar
2 : In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD). PLoS ONE 2013; 8:e58150Crossref, Medline, Google Scholar
3 : Effects of EMDR psychotherapy on 99mTc-HMPAO distribution in occupation-related post-traumatic stress disorder. Nucl Med Commun 2007; 28:757–765Crossref, Medline, Google Scholar
4 : Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med 2007; 37:1481–1491Crossref, Medline, Google Scholar
5 : Effects of psychotherapy on regional cerebral blood flow during trauma imagery in patients with post-traumatic stress disorder: a randomized clinical trial. Psychol Med 2008; 38:543–554Crossref, Medline, Google Scholar
6 : Psychotherapy for military-related posttraumatic stress disorder: review of the evidence. Clin Psychol Rev 2013; 33:45–53Crossref, Medline, Google Scholar
7 : Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry 2006; 60:376–382Crossref, Medline, Google Scholar
8 : Individual differences in recovery from traumatic fear. Trends Neurosci 2013; 36:23–31Crossref, Medline, Google Scholar
9 : The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol 2013; 591:2381–2391Crossref, Medline, Google Scholar
10 : The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 2013; 14:417–428Crossref, Medline, Google Scholar
11 : Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 2012; 13:769–787Crossref, Medline, Google Scholar
12 : Is posttraumatic stress disorder a stress-induced fear circuitry disorder? J Trauma Stress 2009; 22:409–415Crossref, Medline, Google Scholar
13 : The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35:169–191Crossref, Medline, Google Scholar
14 : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Crossref, Medline, Google Scholar
15 : Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 2012; 36:2130–2142Crossref, Medline, Google Scholar
16 : Neural circuitry of PTSD with or without mild traumatic brain injury: a meta-analysis. Neuropharmacology 2012; 62:598–606Crossref, Medline, Google Scholar
17 : Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Res 2008; 162:59–72Crossref, Medline, Google Scholar
18 :
19 : Effects of post-traumatic stress disorder on occipital lobe function and structure. Neuroreport 2012; 23:412–419Medline, Google Scholar
20 : Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychol Med 2013; 43:1533–1542Crossref, Medline, Google Scholar
21 : The effects of temporal unpredictability in anticipation of negative events in combat veterans with PTSD. J Affect Disord 2013; 146:426–432Crossref, Medline, Google Scholar
22 : Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depress Anxiety (Epub ahead of print, Feb 22, 2014)Google Scholar
23 : Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am J Psychiatry 2011; 168:979–985Crossref, Medline, Google Scholar
24 : Neural and behavioral correlates of peritraumatic dissociation in an acutely traumatized sample. J Clin Psychiatry 2012; 73:420–426Crossref, Medline, Google Scholar
25 : The neural basis of flashback formation: the impact of viewing trauma. Psychol Med 2013; 43:1521–1532Crossref, Medline, Google Scholar
26 : An fMRI investigation of posttraumatic flashbacks. Brain Cogn 2013; 81:151–159Crossref, Medline, Google Scholar
27 : The dissociative subtype of posttraumatic stress disorder: rationale, clinical and neurobiological evidence, and implications. Depress Anxiety 2012; 29:701–708Crossref, Medline, Google Scholar
28 : The dissociative subtype of PTSD: a replication and extension. Depress Anxiety 2012; 29:679–688Crossref, Medline, Google Scholar
29 : Posttraumatic stress disorder: a theoretical model of the hyperarousal subtype. Front Psychiatry 2014; 5:37Crossref, Medline, Google Scholar
30 : Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci 2010; 35:258–266Crossref, Medline, Google Scholar
31 : Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med 2012; 74:904–911Crossref, Medline, Google Scholar
32 : Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology 2013; 38:1889–1898Crossref, Medline, Google Scholar
33 :
34 : Premorbid brain volume estimates and reduced total brain volume in adults exposed to trauma with or without posttraumatic stress disorder: a meta-analysis. Cogn Behav Neurol 2010; 23:124–129Crossref, Medline, Google Scholar
35 : Neuroimaging of child abuse: a critical review. Front Hum Neurosci 2012; 6:52Crossref, Medline, Google Scholar
36 : Neuroimaging in children, adolescents and young adults with psychological trauma. Eur Child Adolesc Psychiatry 2013; 22:745–755Crossref, Medline, Google Scholar
37 : Changes in brain anatomy during the course of posttraumatic stress disorder. Psychiatry Res 2011; 193:93–100Crossref, Medline, Google Scholar
38 : Symptoms of posttraumatic stress disorder and exposure to traumatic stressors are related to brain structural volumes and behavioral measures of affective stimulus processing in police officers. Psychiatry Res 2012; 204:25–31Crossref, Medline, Google Scholar
39 : Hippocampal volumes in patients with chronic combat-related posttraumatic stress disorder: a systematic review. J Neuropsychiatry Clin Neurosci 2013; 25:12–25Link, Google Scholar
40 : Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34:1181–1188Crossref, Medline, Google Scholar
41 : Hippocampal volume is inversely related to PTSD duration. Psychiatry Res 2014; 222:119–123Crossref, Medline, Google Scholar
42 : Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry 2013; 73:70–74Crossref, Medline, Google Scholar
43 : Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neurosci Biobehav Rev 2014; 43C:163–172Crossref, Google Scholar
44 :
45 .: Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Res 2012; 203:139–145Google Scholar
46 : Decreased premotor cortex volume in victims of urban violence with posttraumatic stress disorder. PLoS ONE 2012; 7:e42560Crossref, Medline, Google Scholar
47 : Volumetric analysis of amygdala, hippocampus, and prefrontal cortex in therapy-naive PTSD participants. Biomed Res Int (Epub ahead of print, March 13, 2014)Google Scholar
48 : Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002; 5:1242–1247Crossref, Medline, Google Scholar
49 : Twin studies of posttraumatic stress disorder: differentiating vulnerability factors from sequelae. Neuropharmacology 2012; 62:647–653Crossref, Medline, Google Scholar
50 : Regional cerebral volumes in veterans with current versus remitted posttraumatic stress disorder. Psychiatry Res 2013; 213:193–201Crossref, Medline, Google Scholar
51 : Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum Brain Mapp 2013; 34:2808–2816Crossref, Medline, Google Scholar
52 : Anatomical deficits in adult posttraumatic stress disorder: A meta-analysis of voxel-based morphometry studies. Behav Brain Res 2014; 270C:307–315Crossref, Google Scholar
53 : Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Arch Gen Psychiatry 2012; 69:1080–1086Crossref, Medline, Google Scholar
54 : Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. Neuroimage Clin 2013; 2:601–611Crossref, Medline, Google Scholar
55 : Reduced cortical thickness in veterans exposed to early life trauma. Psychiatry Res (Epub ahead of print, May 2, 2014)Google Scholar
56 : Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry 2008; 63:550–556Crossref, Medline, Google Scholar
57 : Association between flashbacks and structural brain abnormalities in posttraumatic stress disorder. Eur Psychiatry 2011; 26:525–531Crossref, Medline, Google Scholar
58 : Evidence of diffuse damage in frontal and occipital cortex in the brain of patients with post-traumatic stress disorder. Neurol Sci 2012; 33:59–68Crossref, Medline, Google Scholar
59 : Gray matter volume alterations related to trait dissociation in PTSD and traumatized controls. Acta Psychiatr Scand 2013; 128:222–233Crossref, Medline, Google Scholar
60 : Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. J Clin Psychiatry 2010; 71:1636–1644Crossref, Medline, Google Scholar
61 : Voxel-based morphometric gray matter correlates of posttraumatic stress disorder. J Anxiety Disord 2013; 27:413–419Crossref, Medline, Google Scholar
62 : Changes in brain electrical activity after cognitive behavioral therapy for posttraumatic stress disorder in patients injured in motor vehicle accidents. Psychosom Med 2008; 70:13–19Crossref, Medline, Google Scholar
63 : Neurobiological correlates of EMDR monitoring - an EEG study. PLoS ONE 2012; 7:e45753Crossref, Medline, Google Scholar
64 : Hemodynamic responses of eye movement desensitization and reprocessing in posttraumatic stress disorder. Neurosci Res 2009; 65:375–383Crossref, Medline, Google Scholar
65 : Police officers under attack: resilience implications of an fMRI study. J Psychiatr Res 2011; 45:727–734Crossref, Medline, Google Scholar
66 : Neural correlates of recovery from post-traumatic stress disorder: a longitudinal fMRI investigation of memory encoding. Neuropsychologia 2011; 49:1771–1778Crossref, Medline, Google Scholar
67 : Quantifiable change in functional brain response to empathic and forgivability judgments with resolution of posttraumatic stress disorder. Psychiatry Res 2005; 140:45–53Crossref, Medline, Google Scholar
68 : High-resolution brain SPECT imaging and eye movement desensitization and reprocessing in police officers with PTSD. J Neuropsychiatry Clin Neurosci 2005; 17:526–532Link, Google Scholar
69 : Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci 2007; 18:127–129Crossref, Medline, Google Scholar
70 : Improvement in cerebral function with treatment of posttraumatic stress disorder. Ann N Y Acad Sci 2010; 1208:142–149Crossref, Medline, Google Scholar
71 : Treatment effects on insular and anterior cingulate cortex activation during classic and emotional Stroop interference in child abuse-related complex post-traumatic stress disorder. Psychol Med 2012; 42:2337–2349Crossref, Medline, Google Scholar
72 : Compelling evidence that exposure therapy for PTSD normalizes brain function. Stud Health Technol Inform 2014; 199:61–65Medline, Google Scholar
73 : Effects of psychotherapy on hippocampal volume in out-patients with post-traumatic stress disorder: a MRI investigation. Psychol Med 2005; 35:1421–1431Crossref, Medline, Google Scholar
74 : EMDR treatment for posttraumatic stress disorder, with focus on hippocampal volumes: a pilot study. J Neuropsychiatry Clin Neurosci 2011; 23:E1–E2Link, Google Scholar
75 : Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol Psychiatry 2013; 74:793–800Crossref, Medline, Google Scholar
76 : Functional neuroimaging of treatment effects in psychiatry: methodological challenges and recommendations. Int J Neurosci 2012; 122:483–493Crossref, Medline, Google Scholar



