Mild Cognitive Impairment and Risk of Mortality in HIV-1 Infection
Abstract
HIV-1–associated cognitive impairment has only been preliminarily investigated for associations with mortality. The authors examined 119 HIV-1–positive homosexual men (asymptomatic: n=96; early symptomatic: n=23). At follow-up (to 3.5 years), there were 105 survivors and 14 nonsurvivors. Those at the 25th percentile in response speeds and in long-term memory retrieval accuracy were at 6.4 (P<0.02) and 3.5 (P<0.05) times increased mortality risk, respectively, of those at the 75th percentile—independent of baseline CDC clinical stage, CD4 cell count, hemoglobin level, antiretroviral and prophylactic medication use, and sociodemographics. Cognitive impairment should be identified early—for maximization of both functional status and survival time.
The most frequent neuropsychiatric complication of the human immunodeficiency virus–type 1 (HIV-1) infection is cognitive impairment, which may range in severity from a mild cognitive disorder to a severe dementing illness.1–3 Currently, more is known about the clinical significance of the moderate to severe cognitive dysfunction that occurs most frequently during the late stages of HIV-1 infection than is known about the minor impairment that occurs in some individuals during the asymptomatic and early symptomatic stages of this disease. However, some markers have been identified that predict the development of an HIV-1–associated dementia (HAD). McArthur et al.4 reported that increased age and decreased hemoglobin levels (<10.4 g/dl) at the time of diagnosis of AIDS were associated with an increased risk for the development of HAD. In addition, Chiesi et al.5 showed that intravenous substance use and female gender were risk factors for HAD, as well as older age and decreased hematocrit. McArthur et al.4 observed that HAD, in turn, was associated with a median survival time of 6 months, which was shorter than that for those without HAD. Further, Justice et al.,6 using the Yale staging system, found that any neurological deficit (including dementia) predicted decreased survival at 1 year, along with decreased hematocrit (<30%), among other variables. Consequently, clinicians and investigators should become more aware of the significance of identifying HAD in patients with AIDS.
In contrast to HAD, relatively little is known about the clinical importance of the minor cognitive alterations observed in some HIV-1–infected individuals during the asymptomatic and early symptomatic stages of the disease. Further, the reliability of findings of minor cognitive impairment during the asymptomatic stage of HIV-1 infection has been questioned.7–9 The questions are related, in part, to a lack of consistency in the methodology employed and in the interpretation of findings in such studies.10–12 Recently, however, Mayeux et al.13 reported that neuropsychiatric manifestations such as cognitive impairment in HIV-1–infected individuals, prior to the development of AIDS, were significantly correlated with an increased risk of mortality. These findings were independent of peripheral blood laboratory markers for clinical progression in HIV-1 infection, such as the CD4 cell count, as well as red blood cell (RBC) count, and of the clinical stage of HIV-1 infection. It is important to determine whether these findings can be generalized to other samples of HIV-1–infected individuals. The fact that the strain of HIV-1 itself can vary between different populations14,15—for example, in monocyte-macrophage tropism and syncytium induction—may partially account for the differences between studies in whether cognitive deficits are detected during the early stages of HIV-1 infection. The existence of differing strains potentially limits the generalizability of such studies in predicting mortality. If, however, it can be demonstrated that minor cognitive impairment prior to AIDS can predict the increased risk of mortality, that would suggest that such changes in cognition prior to the development of AIDS should not be ignored as artifactual but should be a target instead for therapeutic intervention.
We have previously observed subtle deficits in memory and slowing in information processing speed in a subsample of HIV-1–seropositive homosexual men in the asymptomatic or early symptomatic stages of infection16,17 at the baseline assessment of a longitudinal study. The purpose of this investigation was to determine whether these baseline mild cognitive impairments would be related to an increased risk for mortality by the time of study termination.
METHODS
Subjects
The subjects were 119 homosexual men ages 21 to 58 years in the asymptomatic and early symptomatic clinical stages of HIV-1 infection at entry into a prospective natural history study of HIV-1 infection. Subjects were enrolled between 1987 and 1990, with evaluation at 6-month intervals until their death or until May 1991. Subjects were recruited from a variety of sources, including the University of Miami HIV-1 research screening clinics, community physician referrals, and AIDS-related community-based organizations. Informed consent was obtained after the nature of the procedures had been fully explained.
Exclusion criteria were 1) signs (other than persistent generalized lymphadenopathy) or symptoms referable to HIV-1; 2) CD4 cell count >700 cells/mm3; 3) taking of antiretroviral medications (such as zidovudine, didanosine (ddI), zalcitabine (ddC), stavudine (d4T), or lamivudine (3TC)), immunomodulators, or participation in an HIV-related drug trial at study entry; 4) history of excessive alcohol or substance use; 5) history of severe head trauma associated with loss of consciousness; 6) evidence of HIV-associated CNS disease; or 7) history of a major psychiatric disorder (such as schizophrenia or other psychosis or bipolar affective disorder) prior to HIV-1 infection. A history of major depressive disorder did not warrant exclusion. Although a history of injection drug use was not explicitly employed as an exclusion criterion, no subjects with this history were enrolled (as would be expected per the exclusion for history of excessive alcohol and substance use).
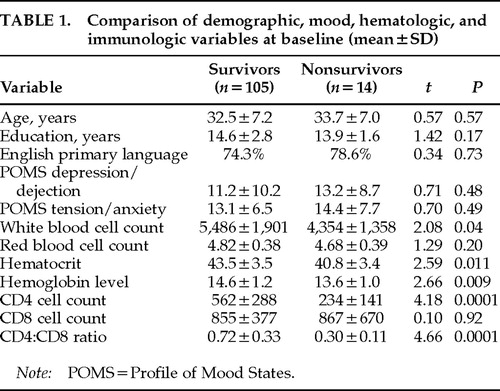
There were 14 deaths over the follow-up period among the 119 HIV-1–seropositive subjects. Table 1 lists the characteristics at study entry for the 105 survivors and 14 nonsurvivors. The survivors were followed for a median 24 months (mean±SD=23.4±14.5; subsample distribution by length of follow-up: <6 months, 13.5%; at least 6 months, 6.7%; 1 year, 9.6%; 1.5 years, 12.5%; 2 years, 11.6%; 2.5 years, 12.5%; 3 years, 16.3%; 3.5 years, 17.3%) and the nonsurvivors for a median 18 months (mean 18.4±8.6; subsample distribution by length of follow-up: <6 months, 7.1%; at least 6 months, 7.1%; 1 year, 14.3%; 1.5 years, 35.8%; 2 years, 21.5%; 2.5 years, 7.1%; 3 years, 7.1%; 3.5 years, 0%). All of the deaths were HIV-1 related. The two groups were similar with respect to age, education, and primary language. They were also similar in clinical stage, as measured by the 198618 or the 199319 Centers for Disease Control and Prevention (CDC) clinical staging systems. On average, the subjects were in their early 30s and had 14 to 15 years of education. Seventy-one percent of the nonsurvivors and 75% of the survivors spoke English as their primary language. The remaining subjects within each group were second- or third-generation Cuban Americans who were bilingual but spoke fluent English. The groups were within normal limits and did not differ on measures of anxious and depressive mood symptoms as measured by the Profile of Mood States tension-anxiety and depression-dejection subscales.20
On initial evaluation, 75% of both the survivors and the nonsurvivors were asymptomatic or had persistent generalized lymphadenopathy (PGL) and were at the 1986 CDC stages II or III (n=96; 1993 CDC clinical stage A—asymptomatic). The remaining 25% in both groups were at the 1986 CDC stage IVA (constitutional symptoms), B (neurological symptoms—all of which were peripheral neuropathies and were non–AIDS-defining) or C-2 (non–AIDS-defining opportunistic infections such as thrush and oral hairy leukoplakia; 1993 CDC clinical stage B [early symptomatic], n=22). No subjects were at 1986 stage IVC-1 (AIDS-defining opportunistic infection) or IVD (tumor) at entry. That is, none were at 1993 CDC clinical stage C (late symptomatic—i.e., clinically defined AIDS). 1993 CDC staging combined clinical staging (A, B, and C, as above) with a numbered immunological staging axis: 1≥500 cells/mm3 (n=54; A1=48, B1=6, C1=0); 2=200–499 cells/mm3 (n=52; A2=40, B2=12, C2=0); 3≤200 cells/mm3 (n=12; A3=8, B3=4, C3=0). Although the 1993 AIDS case definition19 included any individuals with a CD4 cell count <200 cells/mm3 as an AIDS case regardless of clinical stage, it was nevertheless decided to use CD4 cell count here as a continuous rather than a stratified covariate in the analysis to maximize the information available from this control (see statistical methods). Compared with the survivors (mean=562 cells/mm3), as expected, the nonsurvivors did have a significantly lower CD4 lymphocyte count (mean=234 cells/mm3; t=4.18, P=0.0001) and a lower CD4:CD8 ratio (survivors, mean=0.72, vs. nonsurvivors,mean=0.30; t=4.66, P=0.0001; see statistical methods section for the method of controlling this significant difference in the analysis of mortality). They also had significantly lower white blood cell counts, hematocrit levels, and hemoglobin levels than the survivors (see Table 1).
Although none of the subjects were taking zidovudine when enrolled, during the longitudinal follow-up period 32.6% of the survivors and 71.4% of the nonsurvivors had started zidovudine therapy. Other antiretroviral drugs were largely unavailable throughout the trial period. ddI was started by only 3 subjects (2 in combination with zidovudine), ddC by 1 subject (in combination with zidovudine), and d4T and 3TC by no subjects during the follow-up period. The protease inhibitors were not available during the study period. Prophylactic medications for Pneumocystis carinii pneumonia (such as trimethoprim/sulfamethoxazole, aerosolized pentamidine, and dapsone), were started by 22 subjects; for cryptococcal meningitis, by 2 subjects (fluconazole); and for tuberculosis, by 3 subjects (isoniazid; INH). No subjects started rifabutin for Mycobacterium avium intracellulare during the study period.
Cognitive Measures
The cognitive battery consisted of a range of traditional neuropsychological measures as well as information processing speed tasks widely used in experimental cognitive psychology and described in detail elsewhere.16,17 The cognitive domains consisted of measures of 1) language (Wechsler Adult Intelligence Scale–Revised [WAIS-R] Vocabulary,21 Boston Naming Test,22 and Controlled Oral Word Association test [COWA]23); 2) short-term and long-term verbal and visual memory processes (Buschke Selective Reminding Test24 and Logical Memory and Visual Reproductions from the Wechsler Memory Scale [WMS]25); 3) visuospatial/visuoconstructive skills (WAIS-R Block Design21); 4) simple visual reaction time;16 and 5) information processing speed (Posner Letter Matching Task to assess the speed of access to overlearned information26 and response speed during a continuous paired associates learning task under focused and divided attention conditions using a secondary task paradigm27). One-year test-retest reliabilities ranged between 0.50 and 0.82, with the majority greater than 0.70.
Psychosocial Measures
The Profile of Mood States (POMS)20 is a 65-item self-report inventory with several subscales quantifying various mood states—including depressive and anxious mood states, which were used as covariates in our analyses.
Laboratory and Clinical Health Measures
A measure of immunosuppression (CD4 lymphocyte count), oxygen-carrying capacity (venous hemoglobin level), and clinical staging (CDC 198618; CDC 199319) were used as covariates because of their association with disease progression generally and cognitive impairment specifically. Nutritional status (vitamin B6 and B12 levels) was also examined as a covariate in our analyses because of its effect on cognition in HIV-1 infection.28–30 After a 6-hour fast, a total of 50 ml of venous blood was drawn between 8:00 and 10:00 a.m. for immune and nutritional assays. HIV-1–positive serostatus was defined by a doubly reactive enzyme-linked immunosorbent assay (ELISA; Abbott, Chicago, IL) confirmed by Western blot (Hillcrest Laboratories; Cypress, CA). The CD4 cell count was determined by direct immunofluorescence, using a whole-blood staining technique. Briefly, we used a two-color protocol, using an Epics Elite flow cytometer (Coulter Corp, Hialeah, FL), as described by Fletcher and co-workers31,32 The complete blood count (CBC), determined by Cell Dyne 1500 (Sequoia-Turner, Mountain View, CA) with a differential count, provided a measure of the total white blood cell count, the total lymphocyte count, and a hemoglobin level. Percentage of positively stained cells for the CD4 marker was determined by use of Quadstat software (Coulter Epics). Estimates of absolute counts of cells positive for the CD4 surface marker were determined by multiplying peripheral lymphocyte count by percentage of positive cells for the CD4 surface marker.
Plasma pyridoxine (vitamin B6) levels were determined by an erythrocyte transaminase assay,33 and plasma cobalamin (vitamin B12) levels were established by a radioisotope dilution assay.34
Data Analysis
The Cox proportional hazards model35 was used to determine whether mortality between the fall of 1987 and May 31, 1991 (study termination), could be predicted by baseline neuropsychological test performance level (as opposed to impairment versus nonimpairment). This model incorporated controls for the CD4 cell count and other known predictors of mortality in HIV-1 infection. Hazard models appropriately include all available information about subject survival and control for censoring by differential length of follow-up.
In evaluating the value of the cognitive and other variables in predicting mortality, we estimated the Cox proportional hazard models by using maximum likelihood methods with the Statistical Analysis System (SAS) software.36 The risk ratio (RR) was calculated to compare the scores at the 25th and 75th percentiles within this sample. Preliminary analyses were performed to identify potential confounds, which included sociodemographic variables, depressive and anxious mood symptoms from the POMS,20 an immunologic progression marker (CD4 cell count), red blood cell oxygen-carrying capacity (hemoglobin level), zidovudine use, and CDC staging of HIV-1 infection as established by both the 198618 and the 199319 CDC clinical staging systems. (1993 CDC19 immunological staging by the CD4 lymphocyte count was not included here because the latter was retained as a continuous independent control variable to allow for the maximum range of variation.) To avoid type II error in identifying confounds, variables reaching the P≤0.10 level were included in the final analyses. CD4 cell count and hemoglobin level independently maintained P-values ≤0.10 with mortality. In addition, zidovudine use was significantly and positively associated with mortality.
The cognitive test scores were grouped by cognitive domains determined a priori and verified by using Cronbach's α for internal consistencies, which were in the 0.77 to 0.88 range. We transformed the raw scores for each test to z-scores based on the means and standard deviations for this sample of HIV-1–seropositive subjects and obtained an average z-score for each person for each of the five cognitive domains. Examination of the final z-scores revealed kurtosis due to an increased frequency of low neuropsychological performance levels—to be expected in a sample of HIV-1–infected individuals. Hence, the z-scores were ranked in the statistical analyses. A separate Cox analytical model was estimated for each cognitive domain. The n in each analysis varies because of missing data. The composite scores for each of the cognitive domains showing a significant relationship with mortality were then decomposed, and the constituent scales (cognitive tests) were used as predictor variables in post hoc examinations of the mortality relationship. To examine the effect of impairment in each specific domain, the risk ratio comparing the score at the cutoff for the 25th percentile with that for the 75th percentile and its associated confidence interval was calculated, based on the model estimates derived from the data involving the total sample (N=119).
In our statistical models, mortality as of May 31, 1991 (study termination), was the dependent variable. For each cognitive domain, in addition to the baseline cognitive measure for that domain, the two significant laboratory predictors (CD4 lymphocyte count and hemoglobin level), along with zidovudine treatment, were included in the model. Therefore, the relationships of the cognitive variables to mortality are independent of the levels of these control variables. Controlling for the CD4 lymphocyte count and the hemoglobin level reduced the effect size for zidovudine treatment on mortality.
RESULTS
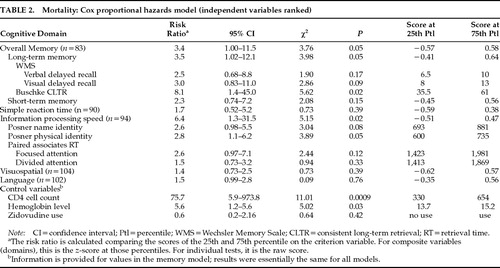
When CD4 lymphocyte count and hemoglobin levels were controlled for, the composite overall memory z-score was significantly associated with mortality (P=0.05), demonstrating that the effect of cognition on mortality was observed in addition to and independently of the effects of CD4 cell count and hemoglobin level. The significant effect of information processing speed was such that those men with the slowest overall information processing speeds were 6.4 times more likely to have died (P=0.02). The results of the analysis of the individual measures comprising the information processing speed domain are presented in Table 2, which indicates that the measures derived from the Posner Letter Matching Task (measuring the speed of access to information in long-term memory) significantly predicted mortality (P=0.02).
When the two letters were judged the same because they were physically identical (e.g., “aa,” “NN“), the response time range was 452—990 ms, median=667, with a cutoff point for the slowest 25% being ≥735 ms (P=0.05), which was –1.02 SD below the mean of the HIV-1–seronegative control group (mean=636±97.5).16 When the two letters were judged the same because they had identical names (e.g., “aA,” “Nn“), the response time range was 495–1,321 ms, median=784 ms, with the slowest 25% cutoff point being ≥881 ms (P=0.08), which was –1.61 SD below the mean of the HIV-1–seronegative control group (mean=712±105).16 In contrast, the measures of the speed of responding during continuous paired associate learning under both focused and divided attention conditions did not predict mortality.
The significant effect of the composite overall memory z-score (P=0.05) was primarily related to the influence of long-term memory rather than short-term memory processes—also depicted in Table 2. The risk ratio shows that HIV-1–seropositive individuals within the lowest quartile in long-term memory scores were 3.5 times more likely to have died within the study period than those at the 75th percentile (P=0.05). An examination of the relationship between the individual measures of long-term memory and mortality showed that the significant effect was predominantly due to performance on the consistent long-term retrieval (CLTR) measure from the Buschke Selective Reminding Task.24 Thus, subjects at the lowest quartile on the CLTR measure were 8.1 times more likely to die than their counterparts scoring higher. The range of CLTR scores was between 12 and 72 words consistently recalled, with a median of 49.5. Those individuals who died were within the lowest quartile with a consistent recall ≤35.5 words, which was –2.10 SD below the mean of the HIV-1–seronegative control group (mean=60.7±12.0).16
Unlike the effects observed in the information processing speed and overall memory domains, the simple reaction time, visuospatial, and language domains did not predict mortality in this sample of subjects.
DISCUSSION
The results of this study in a community-based sample of HIV-1–seropositive homosexual men support and expand on findings presented by Mayeux and colleagues.13 Poor neuropsychological test performance prior to the development of AIDS is a proximal predictor of mortality. The findings herein reveal that minor cognitive alterations in asymptomatic and early symptomatic HIV-1–infected individuals defined by 1986 and 1993 (clinical) staging predicted mortality a median of 18 months prior to death and were mortality predictors over a longer term. The similarity of findings reported by two different research teams at different locations suggests that minor cognitive impairment during the early stages of HIV-1 infection as a marker for mortality is neither a sample- nor a locality-specific phenomenon. Although this study controlled for the effect of CD4 cell count and demonstrated an independent prediction of mortality by cognitive variables, it should nevertheless be pointed out that the mean CD4 cell count of the nonsurvivors in this study was 234 cells/mm3, and caution must be expressed regarding the generalizability of these findings to those with higher CD4 cell counts (for example, those with >500 cells/mm3 or at 1993 CDC immunological stage 1).
The results of this study indicate that a diminished speed of accessing overlearned verbal information from long-term memory and ability to consistently retrieve verbal information from long-term storage were predictive of mortality in this sample. These findings were independent of one another, and both added to the variance accounted for by CD4 lymphocyte count and hemoglobin levels—also significant predictors of mortality. In contrast, measures of language, visuospatial skills, and simple reaction time were not significantly predictive of mortality within the time frame of this study.
An increased risk of mortality was associated with a slower speed of access to semantic memory as well as with deficits in memory due to diminished consistent retrieval from episodic memory. In contrast to Mayeux and colleagues' findings,13 we did not observe that language processes were associated with an increased risk of mortality. HIV-1–associated cognitive alterations have been described as involving primarily subcortical brain processes during the early stages of infection, with language and cortical processes being affected secondarily more in the later stages of infection. The differential latency between involvement of subcortical and cortical structures has been confirmed by PET imaging studies.37 More specifically, it might be postulated that the neuropathophysiological basis for the finding of decreased information processing speed is related to leukoaraiosis (known to be associated with MRI changes of decreased attenuation on T2-weighted images involving deep white matter),38 whereas the finding of decreased memory might be associated with hippocampal neuronal loss (known to occur earlier in HIV-1 brain infection due to a high concentration of CD4 receptor in the hippocampus).39
The finding of Mayeux et al.13 that language was a significant predictor of mortality may relate, in part, to their subjects being at a more advanced stage of HIV-1 infection than those in this study. Mayeux et al. observed that patients' neuropsychological performance was related to mortality at the last visit before their death (or loss to follow-up), controlling for baseline measures. In contrast, this study examined the relationship between neuropsychological performance at the first assessment visit and mortality over the follow-up period. This hypothesis originated with our published observations in 1990 that there was a high level of variability in memory and information processing speed measures leading to significant differences between our HIV-1–seropositive asymptomatic and early symptomatic clinically staged subjects and age- and education-equivalent, HIV-1–seronegative control subjects.16,17
Although the survivors and nonsurvivors differed significantly at baseline in their CD4 lymphocyte counts, they did not differ in clinical stage based on the 198618 or the 199319 CDC clinical staging systems. Further, clinical stage did not remain in the Cox proportional hazards model after the CD4 lymphocyte count was entered, probably because it was accounted for by the CD4 lymphocyte count (which represented a more sensitive control for immunological progression of disease than the cutoff for immunologically defined AIDS19). Zidovudine has generally been associated with an improvement in cognitive functioning and with delay of progression to AIDS, but less consistently with increased survival time.40 At the first assessment visit, none of our HIV-1–seropositive subjects were taking zidovudine, the only FDA-approved antiretroviral agent available at that time. During the longitudinal follow-up period, 32.6% of the survivors and 71.4% of the nonsurvivors started zidovudine therapy. As in the Mayeux et al.13 study, we found a positive relationship between mortality and the use of zidovudine, perhaps because the drug was administered to those HIV-1–seropositive individuals with lower CD4 lymphocyte counts. In a post hoc analysis also controlling for mortality effects due to prophylactic medication for lethal opportunistic infections, we found that prophylactic medication did not account for any additional variance beyond the use of zidovudine and the other controls. In fact, like zidovudine use in our sample, prophylactic medication use was positively related to mortality, probably due to the later stage of disease at the time that these medications were initiated.
Future studies in this area should control for the now more extensive use of other antiretroviral drugs such as ddI, ddC, d4T, 3TC, non-nucleoside reverse transcriptase inhibitors such as nevirapine, and the protease inhibitors (saquinavir, ritonavir, and indinavir)—as monotherapies and in their various combinations—as well as the more extensive use of multiple prophylactic medications for the lethal complications of HIV-1 infection. Regarding the finding of decreased hemoglobin levels, use of erythropoietin (a stimulant of red blood cell production) would also have to be controlled for in future studies. It should be pointed out that we used a sample at an earlier stage of the HIV/AIDS epidemic and that use of prophylactic agents was largely unavailable or very limited at that time, with the predominant exception of Pneumocystis carinii pneumonia prophylaxis and the infrequently required INH prophylaxis for tuberculosis. Hence, use of antiretroviral medications, medication for anemia associated with HIV-1 infection, and prophylactic medications for potentially lethal opportunistic infections did not account for the results herein.
Although we have previously reported that both vitamin B6 and vitamin B12 deficiencies have been observed with cognitive impairment at the baseline assessment in this sample,28–30 controlling for these effects showed no differential effect on survival. However, subjects were notified of their deficiencies by our study and thus may have taken supplements for these vitamins, thereby eliminating their longitudinal impact.30
CONCLUSIONS
As noted by Becker et al.,41 we need to increase our effort to better understand the nature and clinical significance of cognitive deficits in early HIV-1 infection and AIDS. It is therefore especially important to attempt to confirm findings between research centers and in different parts of the country. Our findings, along with those of Mayeux et al.,13 suggest that mild to moderate decrements in cognitive performance observed during the asymptomatic and early symptomatic clinically defined stages of HIV-1 infection are a risk factor for death. Over the length of the follow-up period in this study (up to 3.5 years) and while subjects were clinically defined as asymptomatic or early symptomatic, we found that CD4 lymphocyte count and hemoglobin level predicted mortality. In addition, and independent of these laboratory measures, a slowing in the speed of retrieving overlearned information from semantic memory and a decreased ability to consistently retrieve words from long-term storage also significantly predicted mortality.These findings, taken together, suggest that cognitive dysfunction during the early stages of HIV-1 infection may serve as a marker for early mortality and should be taken into account more broadly in planning therapeutic regimens for HIV-1–infected individuals. This aspect of treatment planning is especially relevant in light of the potential for a future change in the incidence of cognitive impairment among HIV-1–infected individuals. Triple combination antiretroviral medication treatments have frequently reduced HIV-1 load to levels below the limits of assay detection and are deterring the primary progression of HIV-1 infection systemically,40 and there have been demonstrated improved effects of prophylactic medications against the most commonly lethal opportunistic infectious complications of HIV-1. Because of these advances, the survival time for infected individuals will continue to increase. With this longer survival time, it is reasonable to predict that the destructive effects of HIV-1 infection within the brain will have more time to cause neurological and cognitive dysfunction. This possibility may point to the use of pharmacological strategies aimed at reversing the primary pathophysiology of HIV-1 infection in the brain.3 Clinical trials using such agents could determine whether it is possible to alter the relationship observed here between cognitive impairment and increased likelihood of early mortality.
ACKNOWLEDGMENTS
This research was supported in part by Grants 1 PH50 MH/DA42455, MH48628, and MH48628S from the National Institute of Mental Health.
 |
 |
1. Navia BA, Jordan BD, Price RW: The AIDS dementia complex, I: clinical features. Ann Neurol 1986; 19:5Q7–524Google Scholar
2. Grant I, Martin A: Neuropsychology of HIV Infection. New York, Oxford University Press, 1994Google Scholar
3. Goodkin K: The Neuropsychiatric and Behavioral Manifestations of HIV Infection: Section IX, Clinical Manual on HIV and AIDS: An Update. Jacksonville, FL, Florida Medical Association, 1995, pp 113–131Google Scholar
4. McArthur JC, Hoover DR, Bacellar H, et al: Dementia in AIDS patients: incidence and risk factors. Neurology 1993; 43:2245–2252Crossref, Medline, Google Scholar
5. Chiesi A, Vella S, Dally LG, et al: Epidemiology of AIDS dementia complex in Europe. J Acquir Immune Defic Syndr Hum Retrovirol 1996; 11:39–44Crossref, Medline, Google Scholar
6. Justice AC, Feinstein AR, Wells CK: A new prognostic staging system for the acquired immunodeficiency syndrome. N Engl J Med 1989; 320:1388–1393Crossref, Medline, Google Scholar
7. Miller EN, Selnes OA, McArthur JC, et al: Neuropsychological performance in HIV-1 infected homosexual men: the Multicenter AIDS Cohort Study (MACS). Neurology 1990; 40:197–203Crossref, Medline, Google Scholar
8. Selnes OA, Miller E, McArthur J, et al: HIV-1 infection: no evidence of cognitive decline during the asymptomatic stages. Neurology 1990; 40:204–208Crossref, Medline, Google Scholar
9. Wilkins JW, Robertson KR, van der Horst C, et al: The importance of confounding factors in the evaluation of neuropsychological changes in patients infected with human immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retroviol 1990; 3:938–942Medline, Google Scholar
10. Bornstein RA: Methodological and conceptual issues in the study of cognitive changes in HIV infection, in Neuropsychology in HIV Infection, edited by Grant I, Martin A. New York, Oxford University Press, 1994, pp 146–160Google Scholar
11. Newman SP, Lunn S, Harrison MJG: Do asymptomatic HIV-seropositive individuals show cognitive deficit? AIDS 1995; 9:1211–1220Google Scholar
12. White DA, Heaton RK, Monsch AU, et al: Neuropsychological studies of asymptomatic human immunodeficiency virus–type 1 infected individuals. J Int Neuropsychol Soc 1995; 1:304–315Crossref, Medline, Google Scholar
13. Mayeux R, Stern Y, Todak G, et al: Mortality risks in gay men with human immunodeficiency virus infection and cognitive impairment. Neurology 1993; 43:176–182Crossref, Medline, Google Scholar
14. Ayehunie S, Sonnerborg A, Johansson B, et al: Differences in PCR reactivity between HIV proviruses from individuals in Ethiopia and Sweden. J Acquir Immune Defic Syndr Hum Retroviol 1990; 3:975–980Medline, Google Scholar
15. Nowak MA, May RM, Anderson RM: The evolutionary dynamics of HIV-1 quasispecies and the development of immunodeficiency disease. AIDS 1990; 4:1095–1103Crossref, Medline, Google Scholar
16. Wilkie FL, Eisdorfer C, Morgan R, et al: Cognition in early human immunodeficiency virus infection. Arch Neurol 1990; 47:433–440Crossref, Medline, Google Scholar
17. Wilkie FL, Morgan R, Fletcher MA, et al: Cognition and immune function in HIV-1 infection. AIDS 1992; 6:977–981Crossref, Medline, Google Scholar
18. Centers for Disease Control: Classification system for human T-lymphotropic virus type III/lymphadenopathy-associated virus infection. Ann Intern Med 1986; 105:234–237Crossref, Medline, Google Scholar
19. Centers for Disease Control and Prevention: 1993 revised classification system for HIV-1 infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR 1993; 41:1–13Google Scholar
20. McNair D, Lorr M, Doppleman L: EITS Manual for the Profile of Mood States. San Diego, CA, Educational and Industrial Testing Service, 1981Google Scholar
21. Wechsler D: Wechsler Adult Intelligence Scale–Revised. New York, The Psychological Corp, 1981Google Scholar
22. Kaplan E, Goodglass H, Weintraub S, et al: The Boston Naming Test. Philadelphia, Lea and Febiger, 1983Google Scholar
23. Benton AL, Hamsher K: Multilingual Aphasia Examination. Iowa City, IA, University of Iowa, 1976Google Scholar
24. Buschke H, Fuld PA: Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 1974; 24:1019–1025Crossref, Medline, Google Scholar
25. Wechsler D: A standardized memory scale for clinical use. J Psychol 1945; 19:87–95Crossref, Google Scholar
26. Posner MI, Mitchell RF: Chronometric analysis of classification. Psychol Rev 1967; 74:392–409Crossref, Medline, Google Scholar
27. Lansman M, Hunt E: Individual differences in secondary task performance. Memory and Cognition 1982; 10:10–24Crossref, Medline, Google Scholar
28. Beach RS, Morgan R, Wilkie F, et al: Plasma cobalamin levels as a potential cofactor in studies of HIV-1 related cognitive changes. Arch Neurol 1992; 49:501–506Crossref, Medline, Google Scholar
29. Wilkie F, Shor-Posner G, Mantero-Atienza E, et al: Association of vitamin B6 status and reaction time in early HIV-1 infection (abstract). Neurological and Neuropsychological Complications of HIV infection 1991; 2:67Google Scholar
30. Shor-Posner G, Morgan R, Wilkie F, et al: Plasma cobalamin levels affect information processing speed in a longitudinal study of HIV-1 disease. Arch Neurol 1995; 52:195–198Crossref, Medline, Google Scholar
31. Fletcher MA, Baron GA, Ashman MR, et al: Use of whole blood methods in assessment of immune parameters in immunodeficiency states. Diagnostic and Clinical Immunology 1987; 5:69–81Medline, Google Scholar
32. Fletcher MA, Azen S, Adelberg B, et al: Immunophenotyping in a multicenter study: the Transfusion Safety Experience. Clin Immunol Immunopathol 1989; 52:38–47Crossref, Medline, Google Scholar
33. Skala JH, Gretz D, Waring PP: An automated continuous flow procedure for simultaneous measurement of erythrocyte alanine and aspartate amino-transferase activities. Nutrition Research 1987; 7:731–741Crossref, Google Scholar
34. Laud KS, Gottlieb C, Wasserman LR, et al: Measurement of serum vitamin B12 levels using radioisotope dilution and coated charcoal. Blood 1965; 36:202–214Google Scholar
35. Cox DR: Regression models and life-tables. Journal of the Royal Statistical Society [B] 1972; 34:187–220Google Scholar
36. SAS Technical Report P-229: SAS/STAT Software: changes and enhancements, release 6.07. Cary, NC, SAS Institute, 1992Google Scholar
37. Rottenberg DA, Moeller JR, Strother SC, et al: The metabolic pathology of the AIDS dementia complex. Ann Neurol 1989; 22:700–706Crossref, Google Scholar
38. Junque C, Pujol J, Vendrell P, et al: Leuko-ariosis on magnetic resonance imaging and speed of mental processing. Arch Neurol 1990; 47:151–156Crossref, Medline, Google Scholar
39. Pert CB, Smith CC, Ruff MR, et al: AIDS and its dementia as a neuropeptide disorder: role of VIP receptor blockade by human immunodeficiency virus envelope. Ann Neurol 1988; 23(suppl):S71–S73Google Scholar
40. Schmitt FA, Dickson LR, Brouwers P: Neuropsychological response to antiretroviral therapy in HIV infection, in Neuropsychology of HIV Infection, edited by Grant I, Martin A. New York, Oxford University Press, 1994, pp 276–294Google Scholar
41. Becker JT, Caldararo R, Lopez OL, et al: Qualitative features of the memory deficit associated with HIV infection and AIDS: Cross validation of a discriminant function classification scheme. J Clin Exp Neuropsychol 1995; 17:134–142Crossref, Medline, Google Scholar



