Selective Pharmacological Activation of Limbic Structures in Human Volunteers
Abstract
Using a pharmacological probe, procaine hydrochloride, the authors elicited consistent and selective activation of anterior limbic and paralimbic structures in normal human volunteers as documented by H215O positron emission tomography. This activation was associated with a range of emotional, somatic, and visceral experiences, often similar to those experienced during the aura of temporal lobe epilepsy. Several subjects also experienced panic attacks. This study confirms that selective anterior limbic/paralimbic activity in normal human volunteers evokes many emotional phenomena as well as common “ill-defined” symptoms observed in clinical conditions. The present combination of procaine challenge and neuroimaging provides a noninvasive procedure to probe the contribution of different anterior limbic and paralimbic structures to normal human emotions and to neuropsychiatric disorders.
Papez1 and MacLean2 proposed the idea of a limbic system consisting of phylogenetically older brain structures arranged in a network at the center of the brain. These were presumed to be responsible for the subjective and physiological correlates of emotions. The concept has been widely—and often uncritically—adopted by psychiatrists, neurologists, and basic neuroscientists, probably because of its remarkable face validity. Indeed, the split between “cortical” and “limbic” provides an anatomical metaphor for the universal human experience of a distinction between “cognition” and “emotion,” and between “conscious” and “unconscious.” It also seems to explain many puzzling dissociations between preserved cognitive functioning, on the one hand, and impaired emotional or social functioning, on the other, as in patients who suffer from selective damage to the amygdalae/parahippocampal regions3 or to orbitofrontal cortex.4,5
However, it has been pointed out that the concept of a limbic “system” is poorly defined and that evidence for a role in emotions is often circular.6–8 For example, whether a brain area is assumed to be part of the so-called system often depends on whether it is found to be associated with emotions. In addition, some areas, such as the hippocampus, that are clearly “limbic” on anatomical grounds have been found to be primarily related to cognitive rather than emotional functions. Furthermore, the empirical evidence relating limbic structures to emotions in humans remains indirect. The most convincing studies involve direct electrical stimulation using depth electrodes chronically implanted in patients with severe intractable epilepsy.9–11 However, experiences reported by such patients may be related to changes in neural circuitry that are secondary to epilepsy. These experiences may also result primarily from the activation of neocortical efferents rather than from activation that remains intrinsic to limbic circuits, as local recordings cannot rule out this possibility. Finally, functional neuroimaging studies using patients with pathological emotional conditions (such as depression) or emotion-arousing stimuli have not supported the idea of an integrated limbic system responsible for all emotional phenomena. Neocortical areas are typically found to be activated as well.12,13
To go beyond the simplistic idea of “emotions” being the expression of a monolithic “limbic system,” it is necessary to determine 1) what responses—emotional or not—are associated with the selective activation of limbic structures in the normal human brain; and 2) what associations exist between particular structures (or groups of co-activated structures) and specific components of the subjective, autonomic, and endocrine aspects of emotional responses.
Following the method described in Ketter et al.,14 we began to address these issues in healthy human volunteers by using a noninvasive (pharmacological) limbic activation procedure coupled with positron emission tomography (PET) for identification of activated structures. Using this procedure, we can correlate subjective reports with confirmed activation of anterior limbic and paralimbic structures. By capitalizing on the variability of responses between consecutive activations in the same subjects, and between different subjects, the procedure can also identify correlations between specific patterns of regional cerebral blood flow (rCBF) changes and specific subjective and physiological responses to the drug challenge.
We used the local anesthetic procaine hydrochloride as a pharmacological probe. In animals, intravenous administration of procaine induces electrical activity in limbic structures while suppressing activity in neocortical structures.15–17 In humans, procaine induces emotional, autonomic, and endocrine responses comparable to those of direct electrical stimulation of the amygdala.17,18 The mechanism through which procaine selectively activates limbic structures is not known, but it may be related to the particular distribution of mu-opiate and acetylcholine receptors6 or corticotropin-releasing hormone receptors18 in limbic and paralimbic structures. It may also be related to the specific histological characteristics of limbic structures (which, for example, make them a specific target of the herpes virus in the central nervous system6).
Limbic stimulation with intravenous procaine is well suited for PET studies because it is a safe procedure in healthy volunteers, produces peak effects within 2 minutes, and is of short duration (about 5 minutes). In the present study, we recorded subjective and physiological responses to procaine HCl in healthy volunteers while simultaneously measuring changes in rCBF using PET imaging of oxygen-15 water.
METHODS
Subjects
Ten healthy volunteers (mean age 20.4 years, SD=3.66; 5 female, 5 male) participated in the study, which was approved by our institutional review board. All participants were screened for absence of a history of seizures or any psychiatric disorder—including substance abuse—by the Structured Clinical Interview for DSM-III-R, nonpatient version.19 Subjects were informed that the purpose of the study was to establish the effects of systemic administration of a local anesthetic on the central and peripheral nervous system, and to record subjective responses. They were informed that they could withdraw from the study at any time, and all subjects provided informed consent.
Procedures
Prior to PET scanning, subjects received two nonblind injections of procaine (first: 0.96 mg/kg; second: 1.84 mg/kg) at our clinical research center to reduce anticipatory anxiety about the drug. This session also served to familiarize subjects with the postinjection interview (described below) and with the types of experiences about which they were to be questioned during the scanning session. Although this procedure compromises the subsequent single-blinding of injections, the effects of procaine are so discriminable that it seemed more important to reduce anxiety—which may have contaminated the experiment—rather than attempt to preserve an illusory blind.
During scanning, each subject received two single-blind injections of placebo (A) and procaine (B; 1.84 mg/kg) at 20-minute intervals in the PET scanner, in ABBA order to control for the effect of time in the scanner. Anticipatory anxiety was kept as constant as possible by keeping subjects blind to the number and order of procaine and placebo injections.
Regional Cerebral Blood Flow:
Preparation for scanning included application of a thermally molded facial mask to minimize head movements in the ECAT 951r/31 scanner; an intravenous catheter in both antecubital fossa to administer the tracer, placebo (saline), and procaine HCl; leads on the index fingers and chest to record electrodermal and heart rate activity, respectively; and a blood pressure cuff on the left arm. A 10-minute transmission scan using three rotating “pin” sources of germanium-68/gallium-68 for the purpose of calculating attenuation factors preceded the blood flow studies. During each scan, subjects rested quietly in the supine position with eyes open and without any sensory stimulation.
Qualitative rCBF was measured by using the bolus H215O technique20 with a 60-second scan begun 20 seconds after an injection of 50 mCi of H215O diluted in approximately 5–7 ml of saline. Each rCBF measure began 2 minutes after injections of procaine or placebo (corresponding to the peak CNS effects of procaine as determined through pilot studies with a different group of subjects). All images were reconstructed to a resolution of 8 mm full width half maximum (FWHM).
Subjective Phenomena:
Five minutes after each procaine or placebo injection, subjects were extensively interviewed to obtain information about affective, cognitive, somatovisceral, and perceptual experiences. Subjective responses were recorded during a 10-minute structured interview after each injection by using a modified version of the Kellner questionnaire.21 Subjects rated their responses on a 6-point scale, where 6 was anchored as “very intense” or, in the case of common experiences such as anxiety, “the most you ever felt like this in your life.” Subjects were explicitly instructed to rate the magnitude of their experiences following each injection and relative to the preinjection period. During this interview, subjects were also asked to describe in detail more qualitative aspects of their experience.
Psychophysiological and Endocrine Measures:
Heart rate and electrodermal activity were recorded continuously, beginning 1 minute prior to drug or placebo injection. Because of technical problems with the recording equipment, electrodermal activity was not analyzed. Blood samples for beta-endorphin and cortisol levels were obtained 5 and 15 minutes, respectively, after the first two injections.
Data Analysis
Regional Cerebral Blood Flow:
PET images were analyzed by using statistical parametric mapping software.22 Images were registered with the use of an algorithm to correct for small head movements.23 Images were then transformed to a stereotactic coordinate system and were standardized to an atlas brain to facilitate intersubject pooling.24,25 Mean rCBF equivalents (normalized to 50 ml·100 ml–1·min–1) were derived per voxel for each condition and adjusted for the confounding effects of whole-brain differences in blood flow within and between subjects by using analysis of covariance.22 Resulting images were smoothed with a three-dimensional Gaussian filter (20 mm FWHM) to reduce high-frequency noise and reduce the effect of individual differences in gyral anatomy. To identify significant rCBF variance across scans, an omnibus F-test was conducted on a pixel-by-pixel basis. Only pixels with significant variance across the four scans were subjected to further analyses. Planned contrasts (t-statistic) were then conducted on a pixel-by-pixel basis, using the mean rCBF value for each scan to evaluate condition effects (activation=procaine−placebo; deactivation=placebo−procaine). Finally, the resulting pixel-by-pixel distribution of t-values was then transformed to z-scores to allow the associated displays to be independent of degrees of freedom. The critical value for alpha was set at P<0.0001.
Subjective Phenomena:
For the purpose of comparing subjective ratings after procaine and after placebo, ratings were collapsed into experiential domains of emotion, cognition, somatic sensation, and perceptual sensations. These data were then pooled across each of the two placebo and procaine conditions and analyzed by using paired t-tests.
Relations Between rCBF and Subjective Phenomena:
To explore correlations between specific subjective ratings and blood flow changes in particular regions of interest (ROIs), subjects' MRIs were first all coregistered to a common reference MRI. ROIs of a fixed circular size (637 square pixels) were then drawn on the common MRI for the amygdala, anterior cingulate gyrus, insular cortex, inferior frontal cortex, and extrastriate visual cortex, bilaterally (10 ROIs in total). The radioactive counts for each scan of each subject were then derived for each of these ROIs ([10 ROIs]×[9 subjects]×[4 scans]=360 individual values). The mean counts from the two occipital ROIs of a given subject served as a denominator to normalize the count from each ROI. Spearman rank correlations could then be calculated between normalized ROI counts and subjective ratings over the 36 scans available.
RESULTS
Regional Blood Flow Changes
Procaine had highly selective effects on anterior limbic and paralimbic structures (Figure 1). It induced bilateral activation (left panel, Figure 1A) of the entire extent of the anterior cingulate gyrus, of insular cortex, and of the region of the amygdalae/parahippocampal gyri. Notably, no neocortical area was significantly activated. There were several areas of “deactivation” (Figure 1A, right panel): left inferior parietal lobule, right thalamus, and bilateral cerebellum. The locations and magnitudes of these changes are listed in Table 1. Using a less restrictive statistical threshold (P<0.01), we observed rCBF changes in other neocortical areas, but all were also areas of deactivation rather than activation (right medial prefrontal cortex, superior occipital gyrus, precentral and postcentral gyri).
In an additional analysis, PET data for 9 of the 10 subjects were mathematically registered to their own structural MRI (one subject refused to participate in the MRI procedure) by using the algorithm and software described by Woods et al.23 One subject's MR image was selected as a reference MR for the group, and each of the other subjects' MR data sets were then registered to this image. After conversion to this common MR, PET data were then analyzed to create pixel-by-pixel maps of the mean differences in blood flow between procaine and placebo conditions, divided by the standard deviation of the changes; in other words, a mapping of effect sizes (Figure 1B). Regions of interest superimposed on these MRIs further confirmed consistent activation of all anterior limbic and paralimbic structures identified in Figure 1A. (Each of these structures was activated in at least 8 of the 9 subjects.) The image resulting from this analysis is displayed in Figure 1B; regions of activation were determined by setting the visible threshold to z≥3.0.
Subjective Experiences
Activation of this anterior limbic/paralimbic network was associated with brief but distinctive and intense subjective experiences. These experiences cut across psychiatric disease entities and are best described as a powerful and overwhelming feeling that something of direct personal meaning is happening to the subject without specific content. Figure 2 displays the subjects' magnitude ratings of subjective experiences in the placebo and procaine conditions across four categories of reports: emotion, cognition, sensory perception, and somatic sensations. The t-tests indicated that procaine significantly increased subjective effects in all four domains (all t>5.90, df=9, P<0.0005). In the affective domain, subjects reported euphoria, anxiety, depression, and fear. Subjects also typically experienced cognitive phenomena such as derealization and inability to concentrate, as well as a variety of sensory and somatic sensations often accompanying anxiety and psychotic states or the aura of partial complex seizures, such as unformed auditory and visual hallucinations.
Across all subjects, all 13 DSM-IV26 panic symptoms were noted in the spontaneous reports following procaine injections. In contrast, only 3 such symptoms were reported following placebo (chills or hot flushes, paresthesias, and derealization; all rated as “mild intensity” or less). Notably, 4 of 10 subjects reported symptoms consistent with panic anxiety that were clinically indistinguishable from a classic panic attack as defined by DSM-IV criteria. These subjects experienced sudden and intense anxiety (“the worst I've ever felt in my life”; “It was a horrible experience”) without a specific object (“I can't say I was afraid of anything in particular. It was like I was going to die”), accompanied by smothering (“like a huge weight on my chest”; “I felt I couldn't breathe”), intense derealization (“Everything seemed like it was far away”; “I felt spacy and dizzy”) and a variety of peripheral sensations (“My face felt very warm”; “My hands were numb”).
To examine if the experience of panic in our experiment was associated with a specific pattern of anterior limbic/paralimbic activation, we conducted an exploratory analysis using four scans from each of the 4 subjects who had a full-blown panic attack and comparing them with four scans from each of 4 other subjects who had no or very little anxiety (less than 2 on our scale ranging from 0 to 6, where 2 is anchored as “slight”). This analysis (Figure 3) showed that subjects who did not panic had greater rCBF change in the tip of the anterior cingulate cortex (bilaterally) as well as in a small area of left inferior frontal cortex (both P<0.0001) after the procaine injection. There were no other significant differences detected between the two groups.
Finally, we searched for a correlation between the subjective ratings of “anxiety,” “calmness,” and “euphoria” and changes in blood flow in four preselected regions bilaterally: anterior cingulate cortex, amygdala, insular cortex, and inferior frontal cortex. As previously reported by Ketter et al.,14 we found a reliable correlation between counts in the left amygdala and anxiety (r=0.41, P<0.02), but not between the right amygdala and anxiety (r=0.17, P<0.3). Similarly, there was a negative correlation between counts in the left amygdala and the self-rating of “calmness” (r=–0.37, P<0.03), but not between the left amygdala and “euphoria” (r=0.05, P=0.78). The right amygdala did not show such a negative correlation with “calmness.” None of the other ROIs tested showed a significant correlation with the subjective ratings.
Autonomic and Endocrine Changes
Although heart rate was increased at 1 minute following procaine injection, this increase was no longer significant at the time of the PET scan (mean increase at time of scan=3.8 beats per minute, SEM=2.8). As found in previous studies, plasma cortisol level was increased after the first procaine injection (mean increase=4.4 μg/dl, SEM=1.7, P<0.05), but the increase in beta-endorphin—a peptide co-released with adrenocorticotropic hormone—was not statistically significant (mean increase=5.8 pmol/l, SEM=4.0).
Functional Connectivity and Functional Specificity
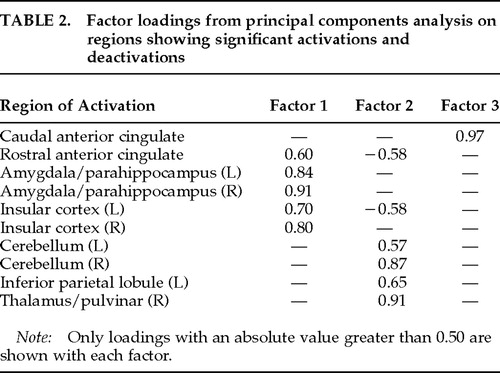
To explore whether the limbic and paralimbic structures exhibiting significant procaine-related effects behaved as a functionally integrated circuit,27 we conducted a varimax-rotated principal components analysis (PCA) on the regions of interest identified in Figure 1. This analysis describes the set of ROIs in terms of uncorrelated (i.e., orthogonal) components that account for a unique amount of variability in the ROIs. Resulting factor loadings reflect the correlations between each ROI and each identified factor. Data were entered into the PCA were the maximal rCBF pixel values from each significant ROI, for each subject, from each of the four scans. Maximal pixel values were taken from rostral and caudal anterior cingulate, given its wide extent and significant evidence of its differential structural and functional organization.28,29 Three factors with eigenvalues ≥1 (the amount of variability accounted for by each component) accounted for 60.5%, 10.7%, and 9.6% of the total variance, respectively. This exploratory analysis revealed three orthogonal patterns of activity (Table 2): ROIs loading primarily on Factor 1 included anterior limbic and paralimbic structures: anterior cingulate, insular cortex, and amygdalae/parahippocampal regions. Factor 2 primarily comprised cerebellum, thalamus, and inferior parietal lobule. Factor 3 loaded most heavily on the caudal aspect of the anterior cingulate. The fact that activations of the anterior limbic/paralimbic structures were more highly correlated with each other than with the nonlimbic ROIs suggests that they behaved as if they were part of a functionally integrated ensemble.
Because there was significant variation in subjective responses between injections and between subjects, we attempted to determine whether specific subjective responses (anxiety, depression, euphoria, perceptual disturbances, derealization) were correlated with different patterns of activation in terms of rCBF. However, in our small sample, this analysis yielded only small and nonsignificant correlations. We suspect that such an analysis would require a larger sample or one in which subjects' mood is controlled experimentally (through mood induction,12 for example) prior to procaine injections.
DISCUSSION
To summarize, the present study demonstrates that selective activation of an anterior limbic and paralimbic network, in the absence of significant neocortical activation, induces powerful emotional and visceral experiences in healthy subjects. Our results confirm the presumption, based on animal and clinical studies, that intense emotions and related somatic and visceral sensations are an important functional correlate of the activation of limbic and paralimbic areas in the normal human brain. Furthermore, the highly selective activation of anterior limbic/paralimbic structures with a pharmacological probe that is distributed diffusely demonstrates that such phylogenetically older brain circuits have distinctly different pharmacodynamics from those of neocortex and that they can be selectively targeted by particular drugs—a fact that has long been suspected from the selective effects of psychiatric medications.
The marked elicitation of somatovisceral phenomena is consistent with suggestions that these sensations are mediated by the insular cortex30 and possibly by part of the cingulate cortex.28 The insula in particular is a highly polymodal region, receiving visceral, olfactory, gustatory, and somatosensory inputs. Although poorly understood, it probably plays an important role in relating interoceptive signals to information from other modalities. Electrical stimulation of the insula has also been specifically associated with gastrointestinal sensations.31,32 Functional imaging studies have only begun to address the function of insular cortex. Activation has been observed in studies of phobic patients stimulated with phobic stimuli13,33 as well as during practice of a cognitive task.34 On the basis of our results, we suspect that insular cortex activation in these other studies reflects the presence of somatovisceral symptoms—either associated with anxious anticipation or with a long, protracted PET scanning session.
We believe that robust activation of the anterior cingulate cortex in our study is related to the motivational and attentional function often attributed to this structure.28,35,36 The anterior cingulate cortex has been implicated in tasks that demand attention (such as the Stroop task) and in the response to motivational states (for example, spontaneous vocalizations as opposed to evoked vocalizations). It is clear that the strong effects of procaine on mood, sensory perception, and somatovisceral sensation powerfully mobilized subjects' attention and could therefore be expected to activate anterior cingulate cortex. (Indeed, one subject graphically described this combined state of attention and somatovisceral sensations as “being grabbed by the balls.”)
Finally, the amygdala has been implicated in emotional responses in general37 and in the fear response in particular.38–40 Given the significant number of subjects who experienced anxiety or fear in response to procaine, it may not be surprising that we observed activation in the amygdalae/parahippocampal region bilaterally. Yet, surprisingly few functional imaging studies have documented amygdala activation in relation to emotional states.
It is important to note that several PET studies of induced emotions have failed to document limbic or paralimbic activation in the presence of very strong emotional responses, including cocaine-induced euphoria and panic anxiety.41–44 These results suggest that limbic or paralimbic activity may be sufficient but not necessary for certain emotional experiences to occur (see, for example, Lane et al.45) or that some emotional experiences do induce activity in such structures, but below the threshold detectable with PET. Such a failure to document limbic or paralimbic activity in studies of induced emotion or of emotional disorders might also reflect a number of methodological problems.46 However, our results significantly overlap with the pattern of rCBF evoked in normal subjects following infusion of the panic-inducing agent cholecystokinin tetrapeptide (CCK4), reported by Benkelfat et al.47 In that study, activation of left anterior cingulate, and of insular cortex and amygdala bilaterally, was observed in the absence of cortical activation.
Both the nature and the variability of the subjective experiences we observed are perhaps easiest to describe in comparison to the clinical manifestations of partial complex seizures (PCS), as previously noted by Stark-Adamec et al.48 These phenomena are also comparable to the responses observed with depth electrode stimulation of limbic structures, such as the amygdala and parahippocampal gyri. Fear is the most common experiential phenomenon described during such experiments.10,11Figure 4 illustrates the similar distribution of subjective experiences in PCS and those following procaine injections in our study. Experiential phenomena during the aura phase of PCS are taken from King and Marsan.49 Only PCS phenomena that were reported in that study and were directly inquired about in our questionnaire were included for comparison. As in that report, emotions refers to subjective feeling of an unexpected and self-limiting emotional state, and visceral sensation refers to localized warmth, pressure, or a rising or sinking feeling experienced inside the chest or abdomen. Cephalic sensations observed were pressure, warmth, tingling, or numbness localized to the head or face. Derealization refers to a feeling of unreality of immediate surroundings. Somatosensory sensations included primarily tingling and numbness but also increased acuity of touch sensations. Visual phenomena were illusions such as changes in light, color vision, or object shadows. Auditory phenomena were unformed hallucinations, typically described as a loud buzzing or ringing heard equally in both ears and an enhancement of auditory acuity. Olfactory symptoms consisted of a hallucination of unpleasant smell (“acrid pungent smell”). Gustatory symptoms consisted of hallucinations of a bitter or metallic taste. The qualitative and quantitative similarity to experiences of epileptic patients was most impressive with respect to emotional and viscerosomatic phenomena. In contrast, procaine may have induced primary sensory illusions and hallucinations more frequently than is typically reported in temporal lobe epilepsy.
The striking similarity of the subjective experiences we recorded and the phenomenology of epileptic auras demonstrates that many of the symptoms experienced by patients with PCS can be a direct consequence of the activation of normal anterior limbic and paralimbic structures, in the absence of an epileptic disease process. We observed these phenomena in almost all of our healthy subjects, none of whom had a history of seizure disorder or psychiatric disorder. Furthermore, the comparable frequency of different subjective phenomena after procaine injection and during PCS auras suggests that anterior limbic stimulation tends to elicit particular symptoms with a comparable frequency independently of the nature of the stimulus (chemical or electrical).
We should note, however, that the emotional phenomena induced by procaine did not include the elicitation of vivid memories often reported by epileptic patients following limbic stimulation. Bancaud et al.9 have suggested that such experiences are related to temporal neocortical activation, which was not observed in our blood-flow maps following procaine injections. Our study therefore tends to support their conclusions about the anatomical origin of vivid memory phenomena in PCS.
The elicitation of panic by intravenous procaine has not been reported previously (except by Ketter et al.14) and has likely been underestimated in the present study, since these symptoms were not directly probed by our questionnaire. Notably, there have been several reports of an association of panic with temporal lobe abnormalities50–52 and with PCS.53–55 There are also reports of successful treatment of panic disorder with limbic anticonvulsants.56 As in Ketter et al.,14 we found a positive correlation between anxiety and blood flow in the left amygdala but not the right. This unilateral finding confirms that, in humans in particular, there may be a lateralization of function between the two amygdalae. Our exploratory analysis of panic versus no-anxiety scans also suggests that subjects who did not experience anxiety may have activated areas of the frontal lobes (tip of the anterior cingulate and left inferior frontal cortex) that could be relevant to inhibiting an anxiety response. Several authors57–59 have proposed that inferior frontal regions contribute to the modulation of emotional responses and that the left frontal cortex in particular is associated with positive affect or inhibition of negative affect.58
A possible concern with our results is that procaine may have a direct vasoconstricting or vasodilating effect on CNS vasculature that could alter activity in the vascular tree directly rather than modify neural activity. Indeed, the areas of activation observed in our study overlap in part with the course of the anterior and middle cerebral arteries. However, there are a number of reasons to believe that the blood flow changes we observed are related to changes in neural activity rather than to vascular effects. First, the impact of an initial bolus of arterial activity on the PET image has been considerably lessened in our study by using the minor modifications in the technique described by Raichle et al.20 The original approach initiates scanning when tracer activity first enters the head (approximately 14–16 seconds after injection), which maximizes the imaging of high arterial activity. Our approach, in contrast, delays onset of scanning until 20 seconds, thus avoiding this initial arterial phase. Also, the scan has been lengthened to 60 seconds from the original 40 seconds, which further dilutes any remaining vascular effect by about 50%. Second, procaine is known to induce electrical activity in limbic structures, as recorded from intracranial electrodes in animals and from intracranial10,11,53 and scalp electrodes in humans.60 Third, the subjective reports of patients were almost identical to those of patients experiencing epileptic activity in limbic structures, and did not include complaints of headaches or nausea as seen in patients with vascular CNS changes.
Our PET deactivation results could suggest that emotional experiences are related to a relative bilateral deactivation of the cerebellum. Cerebellar deactivation has not, however, been implicated previously in emotional experiences, either in healthy volunteers or in psychiatric patients. Our exploratory PCA did not aid in interpreting this cerebellar deactivation, since both the first (anterior limbic and paralimbic structures) and second (cerebellum, inferior parietal lobule and thalamus) factors were correlated with emotional and somatosensory changes associated with procaine (data not shown). However, it is possible that cerebellar deactivation contributed significantly to the marked sense of derealization reported by our subjects following procaine injections. Parietal cortex has been associated with perception of extrapersonal space, and the thalamus is critical to the integration of sensory signals.6 Deactivation of these structures may thus be directly related to the experience of derealization. An alternative hypothesis is that cerebellar deactivation reflects a “steal phenomenon” without any true relationship to derealization. It is also possible that the relative cerebellar deactivation is an artifact of the global normalization process used prior to analysis, which removes the robust increase in whole-brain absolute blood flow seen with procaine.14 Investigation of depersonalization and derealization has received little attention in biological psychiatry, however, and the possibility of a relationship between these symptoms and parietal, thalamic, and cerebellar function may warrant further research.
CONCLUSION
The fact that some anterior limbic and paralimbic structures can be selectively and reliably activated with a pharmacological challenge demonstrates vividly the relative independence of these regions from neocortical circuits. This selectivity can be used to advance the study of how these heretofore poorly understood circuits contribute to behavior and emotional experiences, both normal and abnormal. In particular, it may lead to new diagnostic procedures in neuropsychiatry.61 A combination of functional neuroimaging and a pharmacological limbic “challenge“—whether with procaine or with some other agent—may provide a specific diagnostic procedure for the evaluation of patients with anxiety, mood, or seizure disorders comparable to the thallium “stress test” commonly used to evaluate function in patients suffering from cardiac disease.
ACKNOWLEDGMENTS
The authors thank Drs. J. R. Jennings and C. Gautier for their assistance with the autonomic measures and their helpful comments on this manuscript. This research was supported by funds received from the NIMH Center for Functional Brain Imaging and NIH/NCRR General Clinical Research Center of the University of Pittsburgh (Grant 5M01 RR00056) and an NIMH Research Scientist Development Award and NARSAD Young Investigators Award to the first author, as well as an NIMH Clinical Training in Psychiatry Research Fellowship, a NARSAD Young Investigators Award, and a Scottish Rite Schizophrenia Research Program award to the second author.
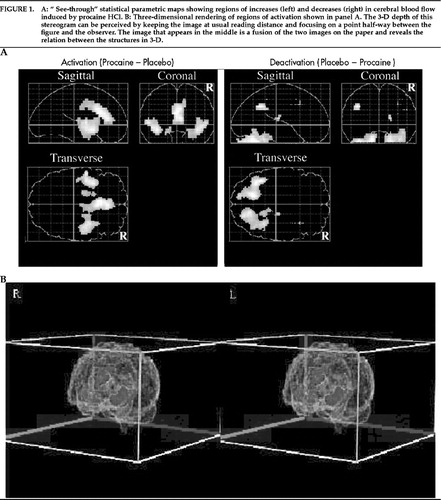
Figure 1. A: “ See-through” statistical parametric maps showing regions of increases (left) and decreases (right) in cerebral blood flow induced by procaine HCl. B: Three-dimensional rendering of regions of activation shown in panel A. The 3-D depth of this stereogram can be perceived by keeping the image at usual reading distance and focusing on a point half-way between the figure and the observer. The image that appears in the middle is a fusion of the two images on the paper and reveals the relation between the structures in 3-D.
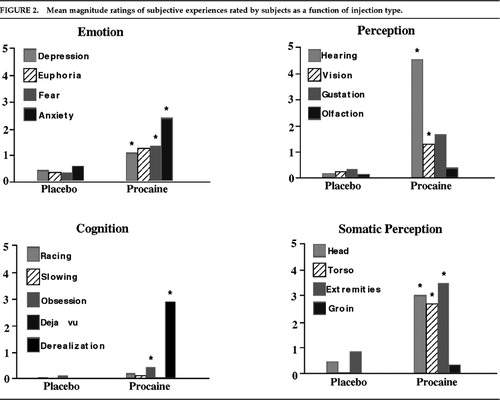
FIGURE 2. Mean magnitude ratings of subjective experiences rated by subjects as a function of injection type.

FIGURE 3. “See-through” statistical maps showing regions of increased cerebral blood flow in subjects who did not experience anxiety following procaine compared with those who experienced a full-blown panic attack.

FIGURE 4. Comparison of the frequency of symptoms induced by procaine with the frequency of occurrence of the same symptoms in a sample of patients with partial complex seizures. Only symptoms rated as “intense” (a rating of 5 or 6) were included in the frequency count.
 |
 |
1. Papez JW: A proposed mechanism of emotion. Arch Neurol Psychiatry 1937; 79:217–224Google Scholar
2. MacLean PD: Psychosomatic disease and the visceral brain: recent developments bearing on the Papez theory of emotion. Psychosom Med 1949; 11:338–353Crossref, Medline, Google Scholar
3. Klüver H, Bucy PC: Preliminary analysis of the temporal lobes in monkeys. Arch Neurol Psychiatry 1939; 42:979–1000Crossref, Google Scholar
4. Blumer D, Benson DF (eds): Personality Changes With Frontal and Temporal Lobe Lesions. New York, Grune and Stratton, 1975Google Scholar
5. Damasio H, Brabowski T, Frank R, et al: The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science 1994; 264:1102–1105Crossref, Medline, Google Scholar
6. Mesulam M-M: Patterns in behavioral neuroanatomy: association areas, the limbic system, and hemispheric specialization, in Principles of Behavioral Neurology, edited by Mesulam M-M. Philadelphia, FA Davis, 1985, pp 1–70Google Scholar
7. LeDoux JE: Emotion and the limbic system concept. Concepts in Neuroscience 1991; 2:169–199Google Scholar
8. Kötter R, Meyer N: The limbic system: a review of its empirical foundation. Behav Brain Res 1992; 52:105–127Crossref, Medline, Google Scholar
9. Bancaud J, Brunet-Bourgin F, Chauvel P, et al: Anatomical origin of déjà vu and vivid “memories” in human temporal lobe epilepsy. Brain 1994; 117:71–90Crossref, Medline, Google Scholar
10. Gloor P, Olivier A, Quesney LF, et al: The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Ann Neurol 1982; 12:129–144Crossref, Medline, Google Scholar
11. Halgren E, Wlater RD, Cherlow DG, et al: Mental phenomena evoked by electrical stimulation of the human hippocampal formation and amygdala. Brain 1978; 101:83–117Crossref, Medline, Google Scholar
12. George MS, Ketter TA, Parekh PI, et al: Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry 1995; 152:341–351Crossref, Medline, Google Scholar
13. Rauch SL, Savage CR, Alpert NM, et al: A positron emission tomographic study of simple phobic symptom provocation. Arch Gen Psychiatry 1995; 52:20–28Crossref, Medline, Google Scholar
14. Ketter TA, Andreason PJ, George MS, et al: Anterior paralimbic mediation of procaine-induced emotional and psychosensory experiences. Arch Gen Psychiatry 1996; 53:59–69Crossref, Medline, Google Scholar
15. Wagman IH, de Jong RH, Prince DA: Effects of lidocaine on the central nervous system. Anesthesiology 1967; 28:155–172Crossref, Medline, Google Scholar
16. Babb TL, Perryman KM, Lieb JP, et al: Procaine-induced seizures in epileptic monkeys with bilateral hippocampal foci. Electroencephalogr Clin Neurophysiol 1979; 47:725–737Crossref, Medline, Google Scholar
17. Adamec RE, Stark-Adamec C, Saint-Hilaire JM, et al: Basic science and clinical aspects of procaine HCl as a limbic system excitant. Prog Neuropsychopharmacol Biol Psychiatry 1985; 9:109–119Crossref, Medline, Google Scholar
18. Kling MA, Gardner DL, Calogero AE, et al: Effects of local anesthetics on experiential, physiologic and endocrine measures in healthy humans and on rat hypothalamic corticotropin-releasing hormone release in vitro: clinical and psychobiologic implications. J Pharmacol Exp Ther 1994; 268:1548–1564Medline, Google Scholar
19. Spitzer RL, Williams JB: Structured Clinical Interview for DSM-III-R. New York, New York State Psychiatric Institute, 1987Google Scholar
20. Raichle ME, Martin WRW, Herscovitch P, et al: Brain blood flow measured with intravenous H2O15, II: implementation and validation. J Nucl Med 1983; 24:790–798Medline, Google Scholar
21. Kellner CH, Post RM, Putnam F, et al: Intravenous procaine as a probe of limbic system activity in psychiatric patients and normal controls. Biol Psychiatry 1987; 22:1107–1126Crossref, Medline, Google Scholar
22. Friston JK, Holmes AP, Worsley KJ, et al: Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping 1995; 2:189–210Crossref, Google Scholar
23. Woods RP, Cherry SR, Mazziotta JC: Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992; 16:620–633Crossref, Medline, Google Scholar
24. Talairach J, Tournoux P: Co-planar Stereotaxic Atlas of the Human Brain. New York, Thieme, 1988Google Scholar
25. Friston KJ, Ashburner J, Poline JB, et al: Spatial realignment and normalization of images. Human Brain Mapping 1995; 2:165–189Crossref, Google Scholar
26. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Press, 1994Google Scholar
27. Friston KJ, Frith CD, Liddle PF, et al: Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab 1993; 13:5–14Crossref, Medline, Google Scholar
28. Devinsky O, Morell MJ, Vogt BA: Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118:279–306Crossref, Medline, Google Scholar
29. Vogt BA, Finch DM, Olson CR: Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 1992; 2:435–443Medline, Google Scholar
30. Mesulam M-M, Mufson EJ: Insula of the old world monkey, III: efferent cortical output and comments on function. J Comp Neurol 1982; 212:38–52Crossref, Medline, Google Scholar
31. Penfield W, Faulx ME: The insula: further observations on its function. Brain 1955; 78:445–470Crossref, Medline, Google Scholar
32. Showers MJC, Lauer EW: Somatovisceral motor patterns in the insula. J Comp Neurol 1961; 117:107–116Crossref, Medline, Google Scholar
33. Drevets WC, Burton H, VIdeen TO, et al: Blood flow changes in human somatosensory cortex during anticipated stimulation. Nature 1995; 373:249–252Crossref, Medline, Google Scholar
34. Raichle ME, Fiez JA, Videen TO, et al: Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex 1994; 4:8–26Crossref, Medline, Google Scholar
35. Pardo JV, Pardo PJ, Janer KW, et al: The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA 1990; 87:256–259Crossref, Medline, Google Scholar
36. Paus T, Petrides M, Evans AC, et al: Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol 1993; 70:453–469Crossref, Medline, Google Scholar
37. Rolls ET: Neurophysiology and functions of the primate amygdala, in The Amygdala, edited by Aggleton JP. New York, Wiley, 1992, pp 143–165Google Scholar
38. Adamec RE: Individual differences in temporal lobe sensory processing of threatening stimuli in the cat. Physiol Behav 1991; 49:455–464Crossref, Medline, Google Scholar
39. LeDoux JE: Sensory systems and emotion. Integrative Psychiatry 1986; 4:237–248Google Scholar
40. LeDoux JE: Brain mechanisms of emotions and emotional learning. Curr Opin Neurobiol 1992; 2:191–197Crossref, Medline, Google Scholar
41. London ED, Cascella NG, Wong DF, et al: Cocaine-induced reduction of glucose utilization in human brain. Arch Gen Psychiatry 1990; 47:567–574Crossref, Medline, Google Scholar
42. Reiman EM, Fusselman MJ, Fox PT, et al: Neuroanatomical correlates of anticipatory anxiety. Science 1989; 243:1071–1074Crossref, Medline, Google Scholar
43. Reiman EM, Raichle ME, Robins E, et al: Neuroanatomical correlates of a lactate-induced anxiety attack. Arch Gen Psychiatry 1989; 46:493–500Crossref, Medline, Google Scholar
44. Fredrikson G, Wik T, Greitz L, et al: Regional cerebral blood flow during experimental phobic fear. Psychophysiology 1993; 30:126–130Crossref, Medline, Google Scholar
45. Lane RD, Reiman EM, Ahern GL, et al: Neuroanatomical correlates of happiness, sadness and disgust. Human Brain Mapping 1995; Suppl 1(abstracts):212Google Scholar
46. Servan-Schreiber D, Perlstein WM: Selective limbic activation and its relevance to emotional disorders. Cognition and Emotion (in press)Google Scholar
47. Benkelfat C, Bradwein J, Meyer E, et al: Functional neuroanatomy of CCK4-induced anxiety in normal healthy volunteers. Am J Psychiatry 1995; 152:1180–1184Crossref, Medline, Google Scholar
48. Stark-Adamec C, Adamec RE, Graham JM, et al: Analysis of facial displays and verbal report to assess subjective state in the non-invasive detection of limbic system activation by procaine hydrochloride. Behav Brain Res 1982; 4:77–94Crossref, Medline, Google Scholar
49. King DW, Marsan CA: Clinical features and ictal patterns in epileptic patients with EEG temporal lobe foci. Ann Neurol 1977; 2:138–147Crossref, Google Scholar
50. Altshuler LL, Devinsky O, Post RM: Depression, anxiety and temporal lobe epilepsy: laterality of focus and symptomatology. Arch Gen Neurol 1990; 47:284–288Crossref, Medline, Google Scholar
51. Edlund MJ, Swann AC, Clothier J: Patients with panic attacks and abnormal EEG results. Am J Psychiatry 1987; 144:508–509Crossref, Medline, Google Scholar
52. Reiman EM, Raichle ME, Butler FK, et al: Focal brain abnormality in panic disorder, a severe form of anxiety. Nature 1984; 310:683–685Crossref, Medline, Google Scholar
53. Devinsky O, Sato S, Theodore WH, et al: Fear episodes due to limbic seizures with normal scalp EEG: a subdural electrographic study. J Clin Psychiatry 1989; 50:28–30Medline, Google Scholar
54. Weilburg JB, Bear DM, Sachs G: Three patients with concomitant panic attacks and seizure disorder: possible clues to the neurology of anxiety. Am J Psychiatry 1987; 144:1053–1056Crossref, Medline, Google Scholar
55. Young GB, Chandarana PC, Blume WT, et al: Mesial temporal lobe seizures presenting as anxiety disorders. J Neuropsychiatry Clin Neurosci 1995; 7:352–357Link, Google Scholar
56. Dantendorfer K, Amering M, Baischer W, et al: Is there a pathophysiological and therapeutic link between panic disorder and epilepsy? Acta Psychiatr Scand 1995; 91:430–432Google Scholar
57. Damasio AR, Van Hoesen GW: Emotional disturbances associated with focal lesions of the limbic frontal lobe, in Neuropsychology of Human Emotion, edited by Heilman KM, Satz P. New York, Guilford, 1983, pp 85–110Google Scholar
58. Davidson RJ: Emotion and affective style: hemispheric substrates. Psychological Science 1992; 3:39–43Crossref, Google Scholar
59. Drevets WC, Raichle ME: Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implications for interactions between emotion and cognition. Cognition and Emotion (in press)Google Scholar
60. Parekh PI, Spencer JW, George MS, et al: Procaine-induced increases in limbic rCBF correlate positively with increases in occipital and temporal EEG fast activity. Brain Topogr 1995; 7:209–216Crossref, Medline, Google Scholar
61. Ryback RS, Gardner EA: Limbic system dysrhythmia: a diagnostic electroencephalogram procedure utilizing procaine activation. J Neuropsychiatry Clin Neurosci 1991; 3:321–329Link, Google Scholar



