Apathy, Depression, and Cognitive Performance in HIV-1 Infection
Abstract
The authors examined the relationship between apathy, depression, and cognitive performance in 48 HIV-1–seropositive and 21 seronegative (control) subjects, using reaction time (RT) and working memory tasks. Apathy, but not depression, was associated with working memory deficits among HIV-seropositive subjects. The cognitive-affective component of the Beck Depression Inventory (BDI), but not apathy, was associated with slowing and decreased accuracy on a choice RT task. The BDI cognitive-affective component was more closely associated than the BDI somatic component with both RT slowing and apathy. Results suggest that prominent symptoms of apathy, independent of depression, may be an important indicator of CNS involvement in HIV infection. Total BDI scores showed a less consistent relationship with neurocognitive performance, suggesting that somatic symptomatology is diagnostically ambiguous among HIV-infected subjects.
Apathy and depression are frequently observed concomitants of central nervous system disease and are especially prominent in disorders affecting the frontal lobes, limbic regions, and subcortical structures.1–3 Human immunodeficiency virus–type 1 (HIV-1) can affect the central nervous system early in the course of illness4–6 and has been shown to exercise an affinity for subcortical structures.7–9 It is therefore not surprising that apathy and depression are both observed, concurrently and independently, in a substantial percentage of HIV-infected patients.10–12
Research has shown that apathy and depression, although they are overlapping dimensions of behavior, can nonetheless be reliably distinguished from one another.2,13 The two syndromes have been observed both alone and concurrently in a variety of neurologic and psychiatric disorders, including Parkinson's disease (PD), Alzheimer's disease, left hemisphere stroke, and negative-syndrome schizophrenia.14–17 Because depression and apathy can occur independently, some investigators have hypothesized that they might have different neurophysiological bases.2,17 Starkstein et al.17 suggested that in PD apathy results from dysfunction in catecholaminergic activity, whereas depression is caused by serotonergic and/or inferior frontal dysfunction. They found that apathetic PD patients (with or without depression) evidenced verbal memory deficits and were slower on speed-dependent tasks than were non-apathetic PD patients. In contrast, depression was associated with less accurate, but not slower, performance on Trails B, a speed-dependent task requiring conceptual set-shifting. That apathy and depression were each associated with unique neuropsychological deficits strengthened this group's conclusion that the two might have different pathophysiologies.
The neuropsychological performance of HIV-infected individuals has been extensively studied, and, clearly, cognitive impairment is consistently observed in this population.18–24 Information processing speed and efficiency seem to be especially affected by HIV infection; a substantial percentage of HIV-seropositive patients show deficits on reaction-time tasks, especially those putting heightened demands on processing capacity.21,25 There are compelling reasons to assume that working memory deficits may result from HIV-1 infection. HIV-1 infection has been demonstrated to affect frontal-subcortical circuits,7–9,26 and current research has implicated frontal-subcortical circuits in the mediation of working memory.27 Gabrieli et al.28 recently concluded that the working memory deficits exhibited in PD are most likely linked to frontal-subcortical circuits mediated by dopamine, a neurotransmitter that has been found to be deficient in HIV-infected patients.29 Additionally, some investigators have suggested that information-processing speed is intimately related to working memory performance,30 and several studies have found decreased processing speed in HIV-seropositive patients.20,21,25,31
Although both neuropsychological impairment and depression occur in HIV-infected patients, research to date suggests that the two do not show a consistent relationship to one another.32–35 Most of this research has ignored the multifactorial nature of depression and the possibility that the somatic symptoms included so prominently in most depression measures might be indicators of physical illness and, therefore, diagnostically ambiguous. Data from our laboratory36 suggest that somatic items included on the Beck Depression Inventory (BDI) are unrelated to memory measures (both procedural and episodic memory), whereas the cognitive-affective items of the BDI are significantly related to procedural memory performance. We have suggested that the cognitive and affective items on the BDI might be more accurate indicators of mood disturbance than the somatic items, especially among medically ill individuals.
There has been a paucity of research examining the role of apathy in HIV-1 infection and none examining the relationship of apathy with depression. Similarly, there has yet to be an empirical study of the relationship between apathy and neuropsychological performance in HIV-1 infection, although there are compelling reasons to suspect a potential relationship. In other subcortical diseases or disorders, the connection between cognitive compromise and apathy has been observed,2,17,37 and a recent case study reported an AIDS patient whose increasing cognitive decline mirrored increasing levels of apathy.38 Also, in a recent review article, Brown39 reported that methylphenidate treatment of cognitive compromise in HIV-1 infection was often found to lead to corresponding changes in affective symptoms, including apathy. Investigators in neurology and neuropsychiatry have suggested that apathy may be indicative of frontal-subcortical disruption1–3 mediated by dopaminergic dysfunction.40 As reported by Gabrieli and colleagues, working memory performance, as measured by neuropsychological tasks such as the Calculation Span, has also been shown to be associated with frontal-subcortical disruption and dopaminergic dysfunction.28
The present study sought to examine the relationship between apathy, depression, information-processing speed, and working memory in a sample of HIV-infected patients and a matched group of seronegative control subjects. In this study, which we believe to be the first empirical study of apathy in HIV-1 infection, we sought to answer four specific questions:
| 1. | Are apathy and depression related to each other in HIV-1 infection, and if so, are they dissociable behavioral domains? | ||||
| 2. | Are apathy and depression more prevalent in HIV-infected patients than in HIV-seronegative control subjects? | ||||
| 3. | Do apathy and depression show a differential relationship to neurocognitive performance among HIV-seropositive patients? | ||||
| 4. | Is working memory performance, which is theoretically related to frontal-subcortical function, more compromised among apathetic than among non-apathetic HIV-seropositive individuals? | ||||
METHODS
Subjects
Sixty-nine subjects drawn from three diagnostic groups participated in the current study: 26 HIV-1–seropositive patients meeting 1993 Centers for Disease Control and Prevention (CDC) criteria for AIDS (CDC Groups C1–3, A3, or B3), 22 HIV-infected subjects who had yet to meet diagnostic criteria for AIDS (pre-AIDS: CDC Groups A1, A2, B1, and B2), and 21 HIV-1 seronegative control subjects. A concerted effort was made to recruit ethnic minority subjects; 11 of 21 control subjects (52%) and 28 of 48 HIV-seropositive patients (58%) were from minority groups. No subject was enrolled who had actual or suspected neurological disease, and any subject with a head injury and subsequent loss of consciousness greater than 5 minutes, seizure disorder, current substance use disorder (substance abuse or dependence), or history of psychosis was excluded. HIV serostatus was ascertained for all subjects by using enzyme-linked immunosorbent assay testing with Western blot confirmation. HIV-seropositive patients were recruited from an infectious disease clinic at a university-affiliated medical center and from a local community organization specializing in serving HIV-infected individuals (AIDS Project Los Angeles). HIV-seronegative control subjects were recruited through newspaper advertisements and through notices posted at both of the above-mentioned sites. The majority of the patients in the current study were gay/bisexual men (52% of control subjects and 69% of patients), and male-male sexual contact was the most common suspected mode of infection with HIV. However, 11 of the 48 HIV-seropositive subjects denied this risk factor and/or suspected some other mode of infection (such as heterosexual sexual contact or injection drug use).
HIV-seropositive participants and HIV-seronegative control subjects did not differ significantly on any demographic variables, including age (P=0.82), education (P=0.19), and premorbid verbal IQ as estimated by the North American version of the National Adult Reading Test (NAART; P=0.31). The mean age of participants was 38.4 years (range 20–63 years), and only 7 of the 69 subjects (10%) did not have at least a high school education (mean=14.5 years, range=8–20 years). HIV-infected subjects reported significantly greater past and current drug use (P<0.03 and P<0.02, respectively), whereas rates of current and past alcohol use were roughly equivalent between the two groups (P=0.21 and P=0.86, respectively). Demographic characteristics of the sample are described in Table 1.
Measures
Depression was measured with the 21-item Beck Depression Inventory, a self-report rating scale that asks about the presence and prominence of cognitive, affective, and somatic symptoms of depression over the past week. Scores on each item can range from 0 (symptom absent) to 3 (presence of symptom is pronounced), yielding a possible range of BDI total scores of 0 to 63. A score of 16 or above is often used to indicate clinically significant depressive symptomatology.41 Because it has been suggested that positively endorsed somatic items on the BDI might be related to physical illness in a medically ill population and therefore be diagnostically ambiguous, we calculated BDI cognitive-affective and BDI somatic subscores, following the procedure outlined in the BDI manual.41 This method of dividing the BDI into subscales, which considers the first 14 items to be cognitive-affective and the final 7 items to be somatic, has been used by other HIV-1 investigators.42,43Table 1 includes means and standard deviations of BDI scores for each subject group.
Apathy was measured with the Neuropsychiatric Inventory (NPI).44 This is a validated brief interview assessing 10 neuropsychiatric symptom domains over the previous 4-week period and is typically administered to the caregivers of patients with neurologic disorders such as Alzheimer's disease or Parkinson's disease. Although the NPI has not been used with HIV-infected patients, it was chosen because it contains an apathy subscale that is easily and briefly administered in interview format (with minor changes in the wording of items to allow for the direct, rather than caregiver, assessment of subjects). The apathy subscale contains seven yes/no questions sampling the domain of apathy (e.g., “Do you feel like you are less spontaneous or less active than usual?” “Are you less likely to initiate a conversation with somebody?” and “Are you less interested in the activities and plans of other people?”), and a subject's apathy score was the total number of positively endorsed items. The item content of this scale is similar to the 18-item Apathy Evaluation Scale designed by Marin, based on the definition of apathy as a simultaneous decrease in the behavioral, cognitive, and emotional concomitants of goal-directed behavior.2
Reaction time (RT) was measured with two computerized tasks. The first was Simple Cued RT (SRT). The SRT task required subjects to respond by pressing a joystick button with the index finger of the dominant hand as quickly as possible after hearing a 1,000-Hz tone. Stimulus onset was always preceded by a visual cue (a red “+” sign displayed for 1,000 ms at the center of the monitor), and the interstimulus interval (ISI) between stimulus cue offset and stimulus onset was either 250, 500, 1,000, or 2,000 ms in duration. ISI was randomly varied. Following administration of a practice block of 8 trials to ensure that the subject fully understood the task, two experimental blocks of 60 trials were administered (each block consisting of 15 trials at each of the four ISIs). The second task was Choice RT (CRT). The CRT task required subjects to rapidly determine whether two sequential visual stimuli were identical. At 1,000 ms following a warning cue (as described above), a complex design subtending 11 degrees vertically and 10 degrees horizontally was presented to the center of the monitor for a duration of 1,000 ms. A second complex design (either identical to or slightly different from the first) was then presented 1,000 ms after offset of the first design. Subjects then vocally responded “same” or “different” into a microphone, with the response detected by a voice-activated relay. Twelve different complex designs were used. Subjects were instructed to respond as quickly as possible without sacrificing accuracy on the visual discrimination task. Following 8 practice trials, two blocks of 30 trials were administered. For both RT tasks, median reaction times were calculated across trials, with responses at less than 125 ms (anticipatory responses) and greater than 1,800 ms (nonresponses) excluded.
Working memory was measured with the Calculation Span (C-SPAN) task. In this task, subjects are orally presented with simple arithmetic problems (e.g., “3+5=…”), which they are instructed to solve by immediately afterward selecting the correct answer from among three written choices while also remembering the last digit in each problem (e.g., “5” in the example above). The number of problems presented on each trial is increased successively from one to nine, with three trials presented at each “level.”45,46 Each level adds one arithmetic problem and thus one additional final digit to remember. The simple math problems had the following characteristics: 1) they were all addition or subtraction with one-digit numbers from 1 to 9; 2) the answers were all positive numbers; 3) the last digit was never the same for successive problems in a trial; and 4) the answer to a math problem is never the same as the target digit that must be remembered. All subjects were required to proceed through all nine “levels” of the C-SPAN task. Three variables were scored on the C-SPAN. The Span score consists of the greatest number of final digits recalled in the correct order for at least two of three trials and can range from 0 to 9. The Trial score is the number of trials in which all final digits were recalled in the correct order and can range from 0 to 27. The Item score corresponds to the total number of final digits reported in the correct order across all 9 levels and can range from 0 to 135. The C-SPAN was added to the study protocol after the beginning of subject testing, and for that reason only 53 of the 69 subjects had complete data for this task.
Procedures
After giving informed consent, all subjects completed the NAART; a detailed demographics questionnaire; the mood, psychotic-spectrum, and substance use disorders modules from the Structured Clinical Interview for DSM-III-R;47 the BDI; and the apathy measure. All diagnostic interviewing was completed by a doctoral-level clinical psychology graduate student (S.A.C.) thoroughly trained in diagnostic interviewing techniques. Subjects then completed all reaction time tasks, including the SRT and CRT tasks detailed above, and finally completed the aforementioned verbal working memory task (the C-SPAN). To minimize any possible effect of fatigue, subjects were instructed to inform the examiner if they felt that they needed breaks in addition to the two mandated 5-minute breaks during the RT tasks. The entire battery (which included several tasks not mentioned above) lasted approximately two hours. Upon completing the study, subjects were paid $25.00 for their participation.
RESULTS
Relationship of Apathy and Depression Among HIV-Seropositive Subjects
To evaluate whether apathy and depression were related to one another among HIV-seropositive subjects, Pearson product-moment correlations were calculated for apathy and depression variables. Depression variables included the BDI total score, as well as subscale scores for the cognitive-affective (BDI-Cog) and the somatic (BDI-Som) components. Among HIV-infected subjects apathy showed a moderately strong correlation with BDI total score (r=0.63, P<0.001), suggesting that in this sample of HIV-seropositive individuals these two scales measure related dimensions. Analysis of the relationship between apathy and BDI-Cog and BDI-Som scores showed that apathy was more strongly associated with BDI-Cog (r=0.67, P<0.001) than with BDI-Som (r=0.33, P=0.03). In an attempt to determine whether those individuals with clinically meaningful levels of depression were more likely to be apathetic, we dichotomized HIV-seropositive patients into groups of those with depression (BDI total score >16: n=21) and those without depression (n=27). An independent-samples t-test comparing apathy scores between these two groups showed that those with clinically meaningful self-reported symptoms of depression had significantly higher apathy scores than did those without (t=5.3, df=47, P<0.001).
Prevalence of Apathy and Depression
Immediately apparent from examination of descriptive data was the near absence of self-reported apathy among control subjects. In fact, the vast majority of HIV-seronegative subjects showed either no or minimal apathy, with only 3 of 21 subjects (14%) endorsing more than 1 item (of the 7 possible items) on the apathy scale. No seronegative subject had a score greater than 3 on the apathy scale, whereas nearly half of all HIV-seropositive patients positively endorsed 5 or more items. The distribution of apathy scores across groups is depicted in Figure 1. To ascertain whether apathy scores differed among the three symptom status groups (HIV-seronegative, Pre-AIDS and AIDS), a univariate analysis of variance (ANOVA) was conducted, which revealed a significant main effect for symptom status (F=10.78, df=2,67, P<0.0001). Tukey post hoc comparisons indicated that both Pre-AIDS and AIDS subjects were significantly more apathetic than the HIV-seronegative control subjects but did not differ from each other (mean±SD, AIDS: 3.6±2.7; Pre-AIDS: 2.9±2.8; HIV-seronegative: 0.52±0.87). An ANOVA comparing BDI total scores among the three symptom groups showed that self-reported depression was also more prominent among HIV-infected subjects than among HIV-seronegative control subjects (F=6.2, df=2,67, P<0.01), again with both seropositive groups differing from control subjects but not from each other (AIDS: 16.4±9.6; Pre-AIDS: 15.6±11.5; HIV-seronegative: 7.4±6.3). Although as a group HIV-seronegative control subjects had lower BDI total scores than did HIV-seropositive patients, it was not uncommon to find clinically significant levels of self-reported depression among HIV-seronegative control subjects. Several HIV-seronegative subjects (5/21; 24%) scored in the range typically considered indicative of at least moderate depression (BDI total score >16).
Relationship of Apathy and Depression to Neurocognitive Performance
To determine whether apathy and depression might be differentially associated with neuropsychological performance in HIV-infected patients, we calculated Pearson correlations for measures of working memory, simple and choice reaction time, apathy, and depression. As can be seen in Table 2, apathy was consistently negatively correlated with working memory performance, but no BDI measure was significantly associated with any of the working memory variables. In contrast, only BDI-Cog was significantly related to CRT performance, with higher scores on this subscale associated with slower and less accurate CRT performance. Among HIV-seropositive patients, somatic symptoms of depression (on BDI-Som) were not related to any neurocognitive variable. Among HIV-seronegative subjects, neither apathy nor depression was significantly associated with neuropsychological performance.
Relationship of Working Memory Performance to HIV Status and Apathy
To examine whether HIV-seronegative, Pre-AIDS, and AIDS subjects differed in working memory performance, three univariate ANOVAs were conducted with symptom status as the grouping variable and C-SPAN variables (Span Score, Trial Score, and Item Score) as dependent variables. A significant group effect was observed for both Item Score (F=6.26, df=2,51, P=0.004) and Trial Score (F=3.18, df=2,51, P=0.05), with Tukey post hoc comparisons revealing that both HIV-seronegative control subjects and Pre-AIDS subjects showed significantly better performance than did AIDS subjects. These results suggest diminished working memory capacity among patients diagnosed with AIDS, but not in mostly asymptomatic HIV-infected individuals.
Because apathy score was significantly associated with all working memory variables, we sought to examine whether apathy might mediate the relationship between disease stage and working memory performance. Analysis of covariance revealed that significant group differences in working memory performance were attenuated when apathy score was accounted for statistically. Group differences on Trial Score were no longer statistically significant (F=1.89, df=3,50, P=0.16), although group differences on Item Score remained significant (F=4.25, df=3,50, P=0.02).
We wished to further explore the association between apathy and working memory performance among HIV-seropositive subjects. As can be seen in Figure 1, HIV-seropositive patients tended to score at either one or the other extreme of the scale on the apathy measure, with 90% of all subjects at either the low or high end of the scale. Twenty-two subjects (45%) had scores of 0 or 1 (Low Apathy) and 22 subjects (45%) had scores of 5, 6, or 7 (High Apathy). Following Starkstein et al.,17 who found a similar bimodal distribution of apathy scores in Parkinson's disease patients, and using the cutoffs described above, we examined whether High Apathy subjects differed from Low Apathy subjects in terms of working memory performance. Independent samples t-tests comparing the High Apathy and Low Apathy groups revealed that the High Apathy subjects were significantly more impaired on all working memory measures than were Low Apathy patients (Span score: t=3.94, df=35, P<0.005; Trials score: t=3.44, df=35, P<0.01; Items score: t=2.52, df=35, P<0.03). These results are depicted in Figure 2. To examine whether the highest- and lowest-scoring patients on the BDI might also show working memory performance differences, we compared the 16 lowest- and 16 highest-scoring HIV-seropositive patients (the top and bottom thirds of our sample) with one another. No differences were noted between High Depression (BDI>20, mean=28.2) and Low Depression (BDI<9, mean=5.1) subjects on working memory variables.
Absolute CD4 count was not correlated with apathy score (P=0.60), nor was it related to apathy group membership (Low Apathy group=318 mm3, High Apathy group=231 mm3). Of the 22 High Apathy subjects, 15 were patients with AIDS, and of the 22 Low Apathy subjects, 11 were patients with AIDS (χ2=1.51, P=0.22).
DISCUSSION
It is perhaps surprising that apathy has not received more empirical scrutiny in HIV-1 research given its prominent role in the clinical presentation of the disease and the disruptive impact it can have on an affected patient's “quality of life.” The results of this study, to our knowledge the first to empirically examine the relationship between apathy and cognitive performance in HIV-1–seropositive subjects, suggest that self-reported symptoms of apathy are quite common among HIV-infected individuals. Although virtually absent in HIV-seronegative control subjects, symptoms of apathy were relatively common in HIV-seropositive patients. This finding suggests that apathy may be specifically associated with the neurologic compromise that often accompanies HIV infection and is not commonly observed in individuals without central nervous system involvement. In contrast, elevated levels of depression were present among both HIV-seropositive and control subjects, with nearly one-fourth of our HIV-seronegative subjects reporting clinically significant levels of depressive symptomatology. This finding underscores the contention that depression is not uncommon among HIV-seronegative subjects. Other groups have similarly reported elevated rates of depression among HIV-seropositive patients relative to HIV-seronegative control subjects.48,49
Our finding that the cognitive-affective subscale of the BDI was more strongly associated with apathy than was the somatic component of the BDI suggests that the overlap between apathy and self-reported depression may be attributable to symptoms from the cognitive and affective domains. This is not surprising given that three BDI cognitive-affective items are quite similar to items on our apathy measure (BDI item 4: life satisfaction/enjoyment; BDI item 12: social interest; BDI item 13: ability to make decisions). Marin et al.13 similarly found that the convergence between a different apathy measure and a different depression measure was primarily due to several similar items between the measures. Clearly, then, the overlap between apathy and depression is related to shared phenomenology between the two, which can be seen by the presence of comparable items on measures of each.
Lending support to the observation that apathy and depression are dissociable affective and behavioral domains is the observation that they share a differential relationship to neurocognitive performance in HIV-seropositive subjects. Apathy, but not depression, was related to working memory impairment; depression, but not apathy, was related to choice reaction time inefficiency. (Depressed patients were less accurate and slower.) This double dissociation between apathy and depression and cognitive performance variables is similar to that observed by Starkstein et al.17 in Parkinson's disease patients, where apathy was associated with verbal memory deficits and depression with less accurate performance on a task requiring conceptual set-shifting. In other studies of HIV-infected subjects, CRT has been shown to be associated with self-reported depression.25,42
The current study suggests that working memory is impaired in a subset of HIV-infected subjects and that these subjects tend to be in the later stages of the disease (i.e., diagnosed with AIDS). This finding is consistent with other studies of working memory in HIV-1 infection.50–52 Consistent with Law and colleagues,50 we did not find compelling evidence of working memory impairment in asymptomatic HIV-infected patients relative to HIV-seronegative control subjects. However, AIDS patients performed significantly worse than HIV-seronegative and Pre-AIDS subjects on two of the three working memory variables, a finding consistent with those of both Sahakian et al.51 and Stout et al.52 Apathy was strongly associated with compromised working memory performance in HIV-infected subjects; when apathy was treated as a covariate, the significant group differences on C-SPAN performance between the AIDS group and the other two participant groups were substantially mediated. Additionally, apathy was significantly correlated with all working memory variables, and High Apathy participants performed significantly worse than Low Apathy subjects on C-SPAN indices. In our opinion, it is unlikely that increased apathy is the cause of poor neuropsychological performance; rather, both apathy and working memory impairment are best conceptualized as related manifestations of frontal-subcortical disruption, perhaps associated with dopaminergic dysfunction. Gabrieli and colleagues,28 in a study using the same working memory task in patients with Parkinson's disease, suggested that dopaminergic deficiency might be contributing to poor working memory performance. HIV infection is in some ways neurochemically similar to PD in that cerebrospinal fluid levels of dopamine or its metabolites have been found to be significantly reduced in some patients with AIDS.29,53
This study suggested that in a sample of HIV-seropositive patients, the somatic factor of the BDI is not related to any cognitive performance variable, whereas the cognitive-affective factor is correlated with both SRT and CRT performance. It is interesting to note that the somatic component of the BDI was not highly correlated with the apathy measure in this study (r=0.33), but the cognitive/affective component of the BDI was strongly associated with apathy score (r=0.67). This dissociation between the somatic and cognitive-affective components of the BDI, a consistent finding across multiple research laboratories,36,42 again calls into question the diagnostic utility of somatic symptoms and signs as indicative of depression in HIV-seropositive patients.
Limitations of the current study must be noted. The relatively small number of participants indicates the need for caution in generalizing to the entire population of HIV-infected patients. We made a concerted effort to include a diverse group of patients and matched the control subjects accordingly, but we did not have the sample size to perform separate analyses on subgroups of theoretical interest (for example, women versus men; heterosexual versus homosexual; injection drug users versus non–iv drug users). It should also be noted that the apathy measure that we used is not as extensive as the Apathy Evaluation Scale developed by Marin.2 It is our belief, however, that the apathy subscale of the NPI, which we used for the current study, contains items that adequately represent the domains mentioned by Marin and covered in his scale. Finally, one must consider the possibility that neuropsychological differences between HIV-infected patients and seronegative control subjects might have been mediated by the patients' greater past and current substance use. Upon closer examination of the self-report data relating to substance use, it was clear that the statistically significant differences between the groups were explained by significantly greater cannabis consumption among HIV-seropositive patients. Past and present intake of other substances was quite similar. Because there is little evidence suggesting that marijuana use influences neuropsychological performance, at least in younger subjects,54 it seems unlikely that group differences in neuropsychological performance are an artifact of substance use.
There were somewhat equivocal findings regarding the link between apathy, depression, and disease progression. CD4 count was unrelated to apathy, depression, or cognitive performance, but there was a tendency for High Apathy subjects to be AIDS (rather than Pre-AIDS) patients. However, the fact that several Pre-AIDS participants were apathetic suggests that apathy is not merely a proxy for disease progression as marked by medical/constitutional status.
Finally, these findings suggest that the presence of apathy is associated with cognitive compromise in HIV-1 infection. Whether apathy contributes to this cognitive compromise or rather merely reflects greater CNS involvement cannot be conclusively determined from the current data. That apathy is related to compromised working memory performance in this sample of HIV-infected patients may reflect a common frontal-subcortical circuit that becomes increasingly compromised with disease progression. Clearly, more research is needed to continue to explore the relationship between apathy and cognitive performance in HIV-1 infection.
ACKNOWLEDGMENTS
The authors thank Nicolette van Sluis, Muriel Veen, and Kim Oostrom for their help with data collection and Dr. Jeffrey L. Cummings for helpful comments on an earlier version of this manuscript. This research was supported in part by grants from the National Institute of Mental Health (1R03 MH54465-01) and the UCLA Academic Senate. Portions of this work were presented at the 25th meeting of the International Neuropsychological Society, Orlando, FL, February 1997.
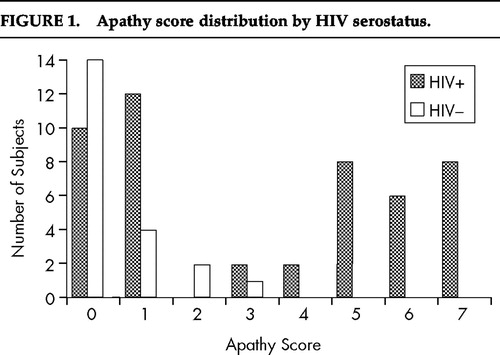
FIGURE 1. Apathy score distribution by HIV serostatus.
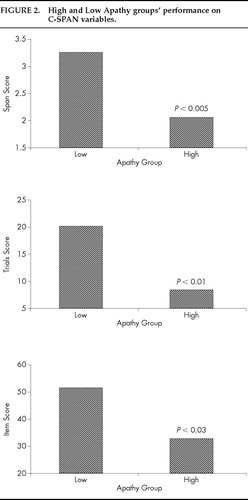
FIGURE 2. High and Low Apathy groups' performance on C-SPAN variables.
 |
 |
1. Cummings JL: Anatomic and behavioral aspects of frontal-subcortical circuits. Ann NY Acad Sci 1995; 769:1–13Crossref, Medline, Google Scholar
2. Marin RS: Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 1991; 3:243–254Link, Google Scholar
3. Strub RL: Frontal lobe syndrome in a patient with bilateral globus pallidus lesions. Arch Neurology 1989; 46:1024–1027Crossref, Medline, Google Scholar
4. Davis LE, Hjelle BL, Miller VE, et al: Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 1992; 42:1736–1739Crossref, Medline, Google Scholar
5. Gray F, Haug H, Chimelli L, et al: Prominent cortical atrophy with neuronal losses correlate of human immunodeficiency virus encephalopathy. Acta Neuropathol 1991; 82:229–233Crossref, Medline, Google Scholar
6. Sonnerborg AB, Ehrnst AC, Bergdahl SK, et al: HIV isolation from cerebrospinal fluid in relation to immunological deficiency and neurological symptoms. AIDS 1988; 2:89–93Crossref, Medline, Google Scholar
7. Navia B, Cho ES, Petito C, et al: The AIDS dementia complex, II: neuropathology. Ann Neurol 1986; 19:525–535Crossref, Medline, Google Scholar
8. Aylward E, Henderer B, McArthur J, et al: Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology 1993; 43:2099–2104Crossref, Medline, Google Scholar
9. Dal Pan G, McArthur J, Aylward E, et al: Patterns of cerebral atrophy in HIV-1–infected individuals: results of a quantitative MRI analysis. Neurology 1992; 42:2125–2130Crossref, Medline, Google Scholar
10. Kernutt G, Price A, Judd F, et al: Human immunodeficiency virus infection, dementia and the older patient. Aust NZ J Psychiatry 1993; 27:9–19Crossref, Medline, Google Scholar
11. Stern R: Neuropsychiatric aspects of HIV, with a note on psychoneuroimmunology. Advances 1994; 10:28–31Google Scholar
12. McArthur J: Neurological and neuropathological manifestations of HIV infection, in Neuropsychology of HIV Infection, edited by Grant I, Martin A. New York, Oxford University Press, 1994, pp 56–107Google Scholar
13. Marin RS, Firinciogullari S, Biedrzycki RC: The sources of convergence between measures of apathy and depression. J Affect Disord 1993; 28:117–124Crossref, Medline, Google Scholar
14. Andreasen NC: Negative symptoms in schizophrenia. Arch Gen Psychiatry 1982; 39:784–794Crossref, Medline, Google Scholar
15. Ott BR, Noto RB, Fogel BS: Apathy and loss of insight in Alzheimer's disease: a SPECT imaging study. J Neuropsychiatry Clin Neurosci 1996; 8:41–46Link, Google Scholar
16. Marin RS, Firinciogullari S, Biedrzycki RC: Group differences in the relationship between apathy and depression. J Nerv Ment Dis 1994; 182:235–239Crossref, Medline, Google Scholar
17. Starkstein SE, Mayberg HS, Preziosi TJ, et al: Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci 1992; 4:134–139Link, Google Scholar
18. Bornstein RA, Pace P, Rosenberger P, et al: Neuropsychological performance in symptomatic and asymptomatic HIV infection. AIDS 1993; 7:519–524Crossref, Medline, Google Scholar
19. Heaton RK, Grant I, Butters N, et al: The HNRC 500-neuropsychology of HIV infection at different disease stages. J Int Neuropsychol Soc 1995; 1:231–251Crossref, Medline, Google Scholar
20. Martin A, Heyes MP, Salazar AM, et al: Progressive slowing of reaction time and increasing cerebrospinal fluid concentrations of quinolinic acid in HIV-infected individuals. J Neuropsychiatry Clin Neurosci 1992; 4:270–279Link, Google Scholar
21. Martin EM, Sorenson DJ, Edelstein HE, et al: Decision-making speed in HIV-1 infection: a preliminary report. AIDS 1992; 6:109–113Crossref, Medline, Google Scholar
22. Selnes OA, Galai N, Bacellar H, et al: Cognitive performance after progression to AIDS: a longitudinal study from the Multicenter AIDS Cohort Study. Neurology 1995; 45:267–275Crossref, Medline, Google Scholar
23. van Gorp WG, Hinkin CH, Satz P, et al: Subtypes of HIV-related neuropsychological functioning: a cluster analysis approach. Neuropsychology 1993; 7:62–72Crossref, Google Scholar
24. Wilkie FL, Eisdorfer C, Morgan R, et al: Cognition in early human immunodeficiency virus infection. Arch Neurol 1990; 47:433–440Crossref, Medline, Google Scholar
25. Miller E, Satz P, Visscher B: Computerized and conventional assessment of HIV-1 infected homosexual men. Neurology 1991; 42:1736–1739Google Scholar
26. Kelly MD, Grant I, Heaton RK, et al: Neuropsychological findings in HIV infection and AIDS, in Neuropsychological Assessment of Neuropsychiatric Disorders, edited by Grant I, Adams KM. New York, Oxford University Press, 1996, pp 403–422Google Scholar
27. Williams MS, Goldman-Rakic PS: Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 1995; 376:572–575Crossref, Medline, Google Scholar
28. Gabrieli JD, Singh J, Strebbins GT, et al: Reduced working memory span in Parkinson's disease: evidence for the role of frontostriatal system in working and strategic memory. Neuropsychology 1996; 10:322–332Crossref, Google Scholar
29. Berger JR, Kumar M, Kumar A, et al: Cerebrospinal fluid in HIV-1 infection. AIDS 1994; 8:67–71Crossref, Medline, Google Scholar
30. Salthouse TA, Babcock RL, Shaw RJ: Effects of adult age on structural and operational capacities in working memory. Psychol Aging 1991; 6:118–127Crossref, Medline, Google Scholar
31. Law WA, Mapou RL, Roller TL, et al: Reaction time slowing in HIV-1 infected individuals: role of the preparatory interval. J Clin Exp Neuropsychol 1995; 17:122–133Crossref, Medline, Google Scholar
32. Bornstein R, Pace P, Rosenberger H, et al: Depression and neuropsychological performance in asymptomatic HIV infection. Am J Psychiatry 1993; 150:922–927Crossref, Medline, Google Scholar
33. Grant I, Olshen RA, Atkinson JH, et al: Depressed mood does not explain neuropsychological deficits in HIV-infected persons. Neuropsychology 1993; 7:53–61Crossref, Google Scholar
34. Hinkin CH, van Gorp WG, Satz P, et al: Depressed mood and its relationship to neuropsychological test performance in HIV-1 seropositive individuals. J Clin Exp Neuropsychol 1992; 14:289–297Crossref, Medline, Google Scholar
35. Perdices M, Dunbar N, Grunseit AC, et al: Anxiety, depression and HIV related symptomatology across the spectrum of HIV disease. Aust NZ J Psychiatry 1992; 26:560–566Crossref, Medline, Google Scholar
36. Kalechstein A, Hinkin CH, Castellon SA, et al: The effects of depression on episodic and procedural memory in HIV-infected persons. Paper presented at the 24th meeting of the International Neuropsychological Society, Chicago, IL, 1996Google Scholar
37. Starkstein SE, Federoff JP, Price TR, et al: Apathy following cerebrovascular lesions. Stroke 1993; 24:1625–1630Crossref, Medline, Google Scholar
38. McDaniel SJ, Summerville MB: Tic disorder associated with encephalopathy in advanced HIV disease. Gen Hosp Psychiatry 1994; 16:298–300Crossref, Medline, Google Scholar
39. Brown GR: The use of methylphenidate for cognitive decline associated with HIV disease. Int J Psychiatry Med 1995; 25:21–37Crossref, Medline, Google Scholar
40. Marin RS, Fogel BS, Hawkins J, et al: Apathy: a treatable syndrome. J Neuropsychiatry Clin Neurosci 1995; 7:23–30Link, Google Scholar
41. Beck AT: Beck Depression Inventory Manual. San Antonio, TX, Psychological Corporation, 1987Google Scholar
42. Harker JO, Satz P, Jones FD, et al: Measurement of depression and neuropsychological impairment in HIV-1 infection. Neuropsychology 1995; 9:110–117Crossref, Google Scholar
43. Law WA, Martin A, Salazar AM, et al: Symptoms of depression in HIV-infected individuals: etiological considerations. Neuropsychiatry, Neuropsychology, and Behavioral Neurology 1993; 6:181–186Google Scholar
44. Cummings JL, Mega M, Gray K, et al: The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
45. Daneman M, Carpenter PA: Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior 1980; 19:450–466Crossref, Google Scholar
46. Salthouse TA, Babcock RL: Decomposing adult age differences in working memory. Developmental Psychology 1991; 27:763–776Crossref, Google Scholar
47. Spitzer RL, Williams JB, Gibbon M, et al: Instruction manual for the Structured Clinical Interview for DSM-III-R. New York, Biometrics Research, New York State Psychiatric Institute, 1989Google Scholar
48. Cleary PD, van Devanter N, Rogers TF: Depressive symptoms in blood donors notified of HIV infection. Am J Public Health 1993; 83:534–539Crossref, Medline, Google Scholar
49. Kelly JA, Murphy DA, Bahr GR, et al: Factors associated with severity of depression and high-risk sexual behavior among persons diagnosed with human immunodeficiency syndrome. Health Psychol 1993; 12:215–219Crossref, Medline, Google Scholar
50. Law WA, Martin A, Mapou RL, et al: Working memory in individuals with HIV infection. J Clin Exp Neuropsychol 1994; 16:173–182Crossref, Medline, Google Scholar
51. Sahakian BJ, Elliot R, Low N, et al: Neuropsychological deficits in tests of executive function in asymptomatic and symptomatic HIV-1 seropositive men 1995; 25:1233–1246Google Scholar
52. Stout JC, Salmon DP, Butters N, et al: Decline in working memory associated with HIV infection. Psychol Med 1995; 25:1221–1232Crossref, Medline, Google Scholar
53. Larsson M, Hagberg L, Forsmen A, et al: Cerebrospinal fluid catecholamine metabolites in HIV-infected patients. J Neurosci Res 1991; 28:406–409Crossref, Medline, Google Scholar
54. Fletcher JM, Page JB, Francis DJ, et al: Cognitive correlates of long-term cannabis use in Costa Rican men. Arch Gen Psychiatry 1996; 53:1051–1057Crossref, Medline, Google Scholar



