Normal Cognitive Performance in Patients With Chronic Alcoholism in Contrast to Patients With Korsakoff's Syndrome
Abstract
This study investigated which cognitive deficits are associated with chronic alcoholism. Neuropsychological profiles and MRI brain structure volumes of 14 patients with Korsakoff's syndrome, 15 patients with chronic alcoholism, and 16 healthy control subjects were compared. The patients with alcoholism had a normal cognitive performance and normal brain structure volumes. The patients with Korsakoff's syndrome had performance deficits on tests of memory, visuoperceptual, and executive functions, as well as reduced brain structure volumes. The results suggest that the cognitive deficits cannot be ascribed to mere chronic consumption of alcohol. If cognitive deficits are present in patients with chronic alcoholism, this may point to an underlying brain disorder.
Prolonged heavy alcohol consumption in combination with severe thiamin deficiency may lead to Korsakoff 's syndrome. Fundamental to this syndrome are profound anterograde and retrograde amnesia. Impairments of visuoperceptual and executive functions may also be present but are less severe.1 There is some evidence that patients with chronic alcoholism also have deficits of visuoperceptual and executive functions,2,3 but few studies have directly compared the cognitive deficits of the two groups of patients. Nonetheless, this comparison is important because it would clarify which deficits are characteristic of Korsakoff's syndrome and which reflect chronic alcoholism. This knowledge would in turn improve our understanding of the cause of alcohol-related cognitive deficits.
In the classical view, cognitive dysfunctions in alcoholic Korsakoff's syndrome develop following thiamin deficiency.4 Subsequent studies have directed attention to the direct toxic effects of alcohol,5,6 but it is unclear which of the cognitive dysfunctions in alcoholic Korsakoff's syndrome reflect the effects of chronic alcohol intake. For these alcohol-related dysfunctions, the continuity hypothesis could hold, which states that different levels of alcoholism are associated with a continuum of cognitive impairment.7,8 The hypothesis predicts that the degree of cognitive dysfunction is based on the frequency, quantity, and duration of alcohol intake.
Thiamin deficiency presumably causes the pathological abnormalities characteristically found in Korsakoff's syndrome, namely atrophy of subcortical structures, particularly the thalamus and mamillary bodies.1 These abnormalities are assumed to underlie the memory deficit, whereas other more widespread cortical and subcortical atrophy may lead to the nonamnesic cognitive deficits.9,10 It follows that memory deficits as a result of atrophy of the thalamus and mamillary bodies are specifically present in patients with Korsakoff's syndrome and are not found in patients with chronic alcoholism.
In the present study we compared the cognitive profiles of age-, sex-, and education-matched groups of patients with chronic alcoholism, patients with alcoholic Korsakoff's syndrome, and healthy control subjects. We hypothesized that 1) both the patients with chronic alcoholism and the patients with Korsakoff's syndrome would have deficits of visuoperception and executive functions, and 2) the patients with Korsakoff's syndrome, but not the patients with chronic alcoholism, would have memory deficits together with atrophy of subcortical structures. We expected the severity of the memory impairment to be correlated with the extent of the atrophy of these subcortical structures.
METHODS
Subjects
Fourteen patients with Alcohol-Induced Persisting Amnestic Disorder (or Korsakoff's Syndrome; DSM-IV11 291.1), 15 patients with Alcohol Dependence (DSM-IV 303.90), and 16 healthy control subjects participated in the study. Patients were diagnosed according to the DSM-IV criteria by a psychiatrist, independent of neuropsychological test results. The patients were recruited from specialized departments for patients with Korsakoff's syndrome or chronic alcoholism from the Vincent van Gogh Institute for Mental Health in Venray, The Netherlands. All patients were abstinent from alcohol for at least 1 month. The control subjects were recruited via newspaper advertisements. The control subjects were screened by a physician, who obtained a detailed medical history using a semistructured interview and conducted a physical and mental examination. Also, the Dutch version of the Symptom Checklist (SCL-90)12,13 and the Dutch version of the Munich Alcoholism Test (MALT)14,15 were administered. Control subjects were excluded when any of these assessments yielded evidence for either current or past psychiatric illness, including Alcohol Dependence or the use of more than 28 drinks a week (i.e., below the norm of the World Health Organization for abuse of alcohol for men of 35 standard consumptions of alcohol-containing beverages per week16). Exclusion criteria for all subjects were the presence of depression; dementia; diabetic mellitus; liver disease; psychotropic medication; other central nervous system diseases; and cardiac, pulmonary, or endocrine diseases that could affect cognitive functioning. Written informed consent was obtained from all subjects.
Subject characteristics are shown in Table 1. Educational level was measured on an 8-point scale, ranging from primary school to higher vocational training and university degree.17 The three groups were matched for age, sex, and level and number of years of education. One-way analyses of variance (ANOVAs) showed that the groups were similar with regard to age (F=0.20, df=2,42, P=0.82), educational level (F=1.03, df=2,42, P=0.37), and IQ score (F=1.30, df=2,39, P=0.28). To estimate total lifetime alcohol consumption and mean weekly consumption, various liquors were converted into units each given a value of 10 g absolute alcohol (Table 1). To obtain these data we had to rely partly on self-report, which may sometimes be unreliable. There were, however, no indications that there were differences between the two patient groups with regard to the indices of drinking history.
Neuropsychological Assessment
Recall from short-term and long-term memory, visuoperceptual and perceptuomotor functions, and frontal-executive functions were assessed by administering the following neuropsychological tests: Verbal Learning Task (VLT),18 Stroop Color-Word Test (SCWT),19 Concept Shifting Test (CST),20 Letter Digit Substitution Test (LDST), and Word Fluency.21 All neuropsychological assessments were conducted by a neuropsychologist who was blind to the diagnosis.
The VLT was used to evaluate retrieval from short-term and long-term memory. The reading and color naming tasks of the SCWT, the number tracking and letter tracking tasks of the CST (which is a modified version of the Trail Making Test),22 and the LDST, which is a modified version of the Symbol Digit Modalities Test,23 were used to assess visuoperceptual and perceptuomotor functions. The interference task of the SCWT, the number/letter shifting task of the CST, and Word Fluency were used to assess executive functions.
The shortened form of the Wechsler Adult Intelligence Scale24 was administered to the patient groups to obtain a measure of general intelligence, and the shortened form of an equivalent Dutch intelligence test, the Groningen Intelligence Test,25 was administered to the control group for the same purpose.
Magnetic Resonance Imaging
A 3-D volumetric scan (T1-weighted, fast-field echo, TR 24 ms, TE 7 ms, flip-angle 30°, number of averages=2, FOV 230 mm, resolution 256×154) was made with a 1.5-tesla scanner (Gyroscan ACS-II, Philips). The slice thickness was 1.5 mm and the scan axis was coronal, perpendicular to the intercommissural line. Data were transferred to a SUN workstation and measurements were taken by ShowImage (developed at the Department of Medical Physics, Free University, Amsterdam, The Netherlands). The delineation between the third ventricle and the thalamus was made by using a seed function. The cutoff value for cerebrospinal fluid was established visually by the rater and adjusted on each slice. The mamillary bodies were measured in most cases on four consecutive slices, with reference to an anatomical atlas.26 The mamillary bodies appeared on the first slice as a bulge in the floor of the third ventricle. This was on average six slices after the first slice on which the third ventricle was measured. On the next two slices the mamillary bodies had an ovoid shape, and on the last slice they were small thickenings in the floor of the third ventricle or the medial wall of the hypothalamus. The left and right mamillary bodies were measured together.
All measurements were done by one rater. Ten scans were remeasured; the Pearson correlation coefficient between the first and second measurement was 0.98 for the third ventricle and 0.86 for the mamillary bodies. Data from the MRI scans were not available for one subject from each of the three groups.
Statistical Analysis
All analyses were conducted by using the SPSS statistical package for Apple Macintosh (version 6.1). Differences between the groups on the cognitive tests and the MRI measures were analyzed in a between-group design using one-way ANOVA with the Tukey multiple-comparison procedure to analyze pairwise group differences (control vs. Korsakoff subjects; control vs. alcoholic subjects; and alcoholic vs. Korsakoff subjects). All tests were two-tailed.
The percentage of subjects with impaired performance was determined by computing tentative cutoff scores derived from age-, sex-, and education-related standard population norms. The cutoff point was set at more than 1.28 standard deviations below the mean normal performance (i.e., the first decile). For this analysis, performance was clustered in the three domains (memory, executive functions, and perceptuomotor speed) according to the procedure described by van Boxtel et al.27 This was done to improve the robustness of the underlying cognitive construct.
Pearson's correlation coefficients between the cognitive variables and the indices of drinking history and between the cognitive variables and the MRI measures were computed for the three groups separately.
RESULTS
Summary statistics for performance on the cognitive tests for the three groups are shown in Table 2. One-way ANOVA with the Tukey multiple-comparison procedure revealed significant differences between the patients with Korsakoff's syndrome and the control subjects on both immediate and delayed recall on the VLT, on two of the three measures of executive functions (Word Fluency and the interference task of the SCWT), and on one of the five measures of visuoperceptual and perceptuomotor speed, the LDST (see Table 2). The patients with Korsakoff's syndrome had lower scores than the patients with chronic alcoholism on the VLT and Word Fluency and tended to have a poorer performance on the interference task of the SCWT and the LDST. No significant differences were found between the patients with chronic alcoholism and the control subjects on any cognitive measure. All correlations between cognitive performance and indices of drinking history were nonsignificant.
The percentage of subjects with impaired performance (in the first decile of normal performance) is shown in Figure 1. Four patients with Korsakoff's syndrome (29%) had an impairment in all three domains, whereas none of the subjects in the other two groups showed an impairment in all three domains.
The volume of the third ventricle was significantly larger, and the volume of the mamillary bodies significantly smaller, in the patients with Korsakoff's syndrome than in the patients with chronic alcoholism and the control subjects (Table 3). Differences in the volumes of the two structures between the two latter groups were nonsignificant. Figure 2 displays MRI images from a patient with Korsakoff's syndrome, a chronic alcoholic patient, and a matched control subject.
There was a significant inverse correlation between memory performance and third-ventricle volume in the patients with Korsakoff's syndrome (R= –0.61, P<0.05, for immediate recall; R= –0.59, P<0.05, for delayed recall). The other correlations between cognitive test performance and third-ventricle volume in these patients were nonsignificant. In the patients with chronic alcoholism, significant correlations in the expected direction were found between delayed recall and third-ventricle volume (R= –0.58, P<0.05). In both the patients with chronic alcoholism and the control subjects, several of the timed measures of visuoperceptual functions correlated significantly with third-ventricle volume (ranging from R=0.55, P<0.05, to R=0.81,P<0.005). No correlations were found between test performance and volume of the mamillary bodies in any of the groups.
DISCUSSION
Patients With Chronic Alcoholism
The most important finding was that in a well-controlled study, detoxified patients with chronic alcoholism had a normal performance on cognitive tasks, even on timed tasks that have proved to be sensitive to mild cognitive disorders, including age-associated cognitive decline20 and mild head injury.28 On some measures of cognitive speed, these patients as a group performed somewhat poorer than the control group, but the differences did not reach the trend level. These findings suggest that if severe cognitive impairment is present in chronic alcoholic patients, this cannot be ascribed to the mere chronic consumption of alcohol. Instead, the cognitive impairment may point to an underlying brain disorder such as Korsakoff's syndrome. This notion is in accordance with the view that up to now there has been no convincing evidence for the existence of a dementia syndrome that is primarily due to the chronic consumption of alcohol, i.e., alcoholic dementia.29,30
It is possible that our findings do not apply to the whole population of patients with chronic alcoholism, since we studied a select group of patients, those who had been referred to a third-line medical care service to undergo detoxification. Further, in the present study psychiatric or medical comorbidity was controlled for by applying strict exclusion criteria. Since chronic alcoholism is characterized by a higher than normal prevalence of psychiatric and medical comorbidity, such as depression and antisocial personality disorder31,32 and brain trauma and liver disease,33,34 it is possible that cognitive impairment due to comorbid conditions is present in a subset of the chronic alcoholic patients.
Patients With Korsakoff's Syndrome
Our findings indicate that a substantial minority of the patients with Korsakoff's syndrome suffer from a more widespread cognitive impairment than just memory deficit. This finding is in accordance with previous reports.1 These cognitive impairments would appear to be essential features of Korsakoff's syndrome, since they were not present in the patients with chronic alcoholism.
The impairments of executive functions may indicate involvement of the frontal cortex in Korsakoff's syndrome. Frontal involvement is consistent with certain clinical features of the syndrome, such as apathy, loss of insight, and confabulation, which are characteristically found in patients with frontal lobe disorders.35
There was no evidence of the global intellectual decline described by Jacobson and Lishman.10 The general intelligence of the patients with Korsakoff's syndrome was within the normal limits. This does not imply, however, that severe cognitive deterioration cannot occur in patients with chronic alcoholism, since patients with clinical evidence of dementia were excluded from this study. Further, it is possible that our findings apply specifically to male patients with Korsakoff's syndrome; there have been some reports indicating greater alcohol-related cognitive impairment in females,36,37 and most of the patients in our sample were male (12 males, 2 females in the Korsakoff group and 12 males, 3 females in the alcoholic group).
Volumetric Measures and Cognitive Performance
In line with our hypothesis, subcortical pathology, as indicated by a significantly enlarged volume of the third ventricle and a significantly reduced volume of the mamillary bodies, was specific for the Korsakoff group. The widening of the third ventricle presumably reflects atrophy of the thalamus, possibly the dorsomedial nucleus. The strong and selective association of the thalamus with memory performance indicates the importance of this structure to the defective memory function seen in Korsakoff's syndrome. In the patients with chronic alcoholism the volumes of the third ventricle and the mamillary bodies were in the normal range, which is consistent with the intact memory performance of these subjects.
The strong correlations between visuoperceptual and perceptuomotor speed and third-ventricle volume may reflect age-related effects, but the age range of the samples in this study was too limited for us to investigate this issue.
CONCLUSIONS
The finding of a normal cognitive performance in chronic alcoholism suggest that cognitive deficits cannot be ascribed to the mere chronic consumption of alcohol. If cognitive deficits are present in patients with chronic alcoholism, this may well point to an underlying brain disorder, namely Korsakoff's syndrome.
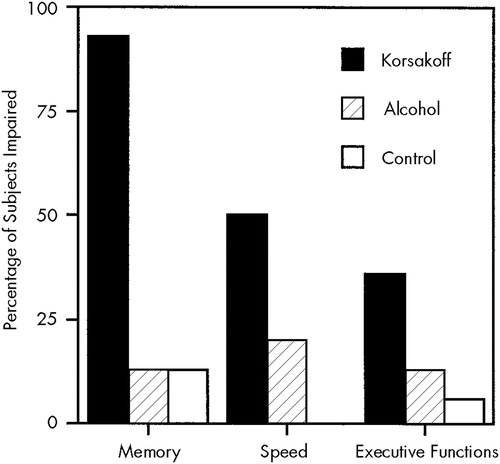
FIGURE 1. Percentage of subjects with impaired performance on memory, speed, and executive functions
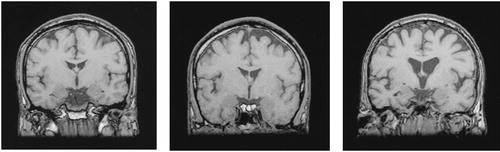
FIGURE 2. Coronal T1-weighted MR images through the level of the third ventricle of a normal control subject (left), a patient with chronic alcoholism (center), and a patient with Korsakoff's syndrome (right)Note the increased volume of the third ventricle in the Korsakoff patient.
 |
 |
 |
1 Kopelman MD: The Korsakoff syndrome. Br J Psychiatry 1995; 166:154–173Crossref, Medline, Google Scholar
2 Beatty WW, Katzung VM, Moreland VJ, et al: Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug Alcohol Depend 1995; 37:247–253Crossref, Medline, Google Scholar
3 Gurling HM, Curtis D, Murray RM: Psychological deficit from excessive alcohol consumption: evidence from a co-twin control study. Br J Addict 1991; 86:151–155Crossref, Medline, Google Scholar
4 Victor W, Adams RD, Collins GH: The Wernicke-Korsakoff Syndrome. Oxford, Blackwell Scientific, 1971Google Scholar
5 Walker DW, Barnes DE, Zorneyzer SF, et al: Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science 1980; 209:711–713Crossref, Medline, Google Scholar
6 Parsons OA, Leber WR: The relationship between cognitive dysfunction and brain damage in alcoholics: causal, interactive, or epiphenomenal? Alcohol Clin Exp Res 1981; 5:326–343Google Scholar
7 Ryback R: The continuum and specificity of the effects of alcohol on memory: a review. Quarterly Journal of Studies on Alcohol 1971; 32:995–1016Google Scholar
8 Butters N: Alcoholic Korsakoff's syndrome: some unresolved issues concerning etiology, neuropathology, and cognitive deficits. J Clin Exp Neuropsychol 1985; 7:181–210Crossref, Medline, Google Scholar
9 Jacobson RR, Lishman WA: Cortical and diencephalic lesions in Korsakoff's syndrome: a clinical and CT scan study. Psychol Med 1990; 20:63–75Crossref, Medline, Google Scholar
10 Jacobson RR, Lishman WA: Selective memory loss and global intellectual deficits in alcoholic Korsakoff's syndrome. Psychol Med 1987; 17:649–655Crossref, Medline, Google Scholar
11 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994Google Scholar
12 Derogatis LR: SCL: Administration, Scoring and Procedures Manual for the Revised Version. Baltimore, Clinical Psychometric Research, 1977Google Scholar
13 Arrindell WA, Ettema JHM: SCL-90: Een multidimensionele psychopathologie indicator [SCL-90: a multidimensional indicator of psychopathology]. Lisse, The Netherlands, Swets & Zeitlinger, 1986Google Scholar
14 Feuerlein W, Kuuml;fner H, Ringer C, et al: Muünchner Alkoholismustest [Munich Alcoholism Test]. Weinheim, Germany, Beltz Testgesellschaft, 1979Google Scholar
15 Walburg JA, van Limbeek J: Muunchen Alcohol Test [Munich Alcoholism Test]. Lisse, The Netherlands, Swets & Zeitlinger, 1987Google Scholar
16 World Health Organization: Problems related to alcohol consumption: report of a WHO expert committee. Geneva, World Health Organization, 1980Google Scholar
17 De Bie SE: Standaardvragen 1987: Voorstellen voor uniformering van vraagstellingen naar achtergrondkenmerken en interviews [Standard questions 1987: proposal for uniformization of questions regarding background variables and interviews], 2nd edition. Leiden, The Netherlands, Leiden University Press, 1987Google Scholar
18 Brand N, Jolles J: Learning and retrieval rate of words presented auditorily and visually. J Gen Psychol 1985; 112:201–210Crossref, Medline, Google Scholar
19 Stroop JR: Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18:643–662Crossref, Google Scholar
20 Houx PJ, Vreeling FW, Jolles J: Age-associated cognitive decline is related to biological life-events, in Alzheimer's Disease: Basic Mechanisms, Diagnosis and Therapeutic Strategies, edited by Iqbal K, McLachlan DRC, Winblad B, et al. Chichester, UK, Wiley, 1991, pp 353–359Google Scholar
21 Lezak MD: Neuropsychological Assessment, 3rd edition. New York, Oxford University Press, 1995Google Scholar
22 Reitan RM: Validity of the Trail Making Test as an indication of organic brain damage. Percept Mot Skills 1958; 8:271–276Crossref, Google Scholar
23 Smith A: The Symbol Digit Modalities Test: a neuropsychological test for economic screening of learning and other cerebral disorders. Learning Disorders 1968; 36:83–91Google Scholar
24 Wechsler D: Wechsler Adult Intelligence Scale Manual. New York, Psychological Corporation, 1955Google Scholar
25 Luteijn F, van der Ploeg FAE: Handleiding Groninger Intelligentietest (GIT) [Manual, Groningen Intelligence Test]. Lisse, The Netherlands, Swets & Zeitlinger, 1983Google Scholar
26 Duvernoy H: The Human Brain: Surface, Three-Dimensional Sectional Anatomy and MRI. Vienna, Springer-Verlag, 1991Google Scholar
27 van Boxtel MPJ, Langerak K, Houx PJ, et al: Self-reported physical activity, subjective health, and cognitive performance in older adults. Exp Aging Res 1996; 22:363–379Crossref, Medline, Google Scholar
28 Klein M, Houx PJ, Jolles J: Long-term persisting cognitive sequelae of traumatic brain injury and the effect of age. J Nerv Ment Dis 1996, 184:459–467Google Scholar
29 Victor M: Alcoholic dementia. Can J Neurol Sci 1994; 21:88–99Crossref, Medline, Google Scholar
30 Joyce EM: Aetiology of alcoholic brain damage: alcoholic neurotoxicity or thiamine malnutrition? Br Med Bull 1994; 50:99–114Google Scholar
31 Penick EC, Powell BJ, Nickel EJ, et al: Co-morbidity of lifetime psychiatric disorder among male alcoholic patients. Alcohol Clin Exp Res 1994; 18:1289–1293Google Scholar
32 Read MR, Penick EC, Powell BJ, et al: Subtyping male alcoholics by family history of alcohol abuse and co-occurring psychiatric disorder: a bi-dimensional model. Br J Addict 1990; 85:367–378Crossref, Medline, Google Scholar
33 Glenn SW, Parsons OA, Stevens L: Effects of alcohol abuse and familial alcoholism on physical health in men and women. Health Psychol 1989; 8:325–341Crossref, Medline, Google Scholar
34 Tarter RE, Arria AM, Van Thiel DH: Hepatic encephalopathy coexistent with alcoholism. Recent Dev Alcohol 1991; 9:205–224Medline, Google Scholar
35 Stuss DT, Benson DF: The Frontal Lobes. New York, Raven, 1986Google Scholar
36 Acker C: Neuropsychological deficits in alcoholics: the relative contributions of gender and drinking history. Br J Addict 1986; 81:395–403Crossref, Medline, Google Scholar
37 Jacobson RR, Acker CF, Lishman WA: Patterns of neuropsychological deficit in alcoholic Korsakoff's syndrome. Psychol Med 1990; 20:321–334Crossref, Medline, Google Scholar



