Relationship Between Symptoms and Motoric Subtype of Delirium
Abstract
For 46 patients with delirium who were consecutive referrals to a consultation-liaison psychiatry service, the authors describe the relationships between symptoms, as rated on the Delirium Rating Scale, and delirium motoric subtypes, as defined by Liptzin and Levkoff's criteria. Most cases were of the mixed subtype (46%), 24% were hypoactive, and 30% were hyperactive. Overall scores differed significantly among motoric subtype groups, being highest in the hyperactive, lowest in the hypoactive, and intermediate in the mixed. On item scores, the hypoactive group scored lower than the hyperactive group for delusions, mood lability, sleep-wake cycle disturbances, and variability of symptoms, but lower than the mixed group only for mood lability. The results suggest that delirium presents as motoric subtypes that differ according to symptom profile and severity of delirium. These subtypes may differ in their underlying pathophysiologies, responsiveness to therapeutic interventions, and outcome.
The syndrome of delirium is commonly encountered in all hospital settings. Patients with delirium tend to require longer hospital stays and have higher mortality rates than nondelirious patients, even when the severity of their underlying illness is accounted for.1,2 Nevertheless, little is known about the neuropathophysiology of this condition. This lack partly reflects the fluctuating nature and variety of underlying etiologies of delirium, but it also reflects the limitations in our understanding of the neurophysiology of the normal functioning brain and the shortcomings of currently available investigative strategies.3
Study of the phenomenology of delirium has also been limited. Most attention has been given to motoric subtypes. The recent emergence of operationalized diagnostic criteria4,5 and standardized symptom rating scales and interviews6,7 has opened the possibility of more detailed investigation of the phenomenology of delirium. The presence of certain delirium symptom or subtype profiles may have implications for prognosis. For example, the duration of episodes of delirium can be distinguished by the severity of certain items on the Delirium Rating Scale (DRS).8
Two studies of delirium subtypes based on psychomotor activity levels9,10 have found that patients with hyperactive phenomenology had shorter hospital stays and better outcomes than either mixed or hypoactive subtypes. In a similar study, Meagher et al.11 found a number of associations between phenomenologic subtypes and ward management practices, including more frequent use of psychotropic medications and environmental manipulations in hyperactive cases. Hyperactive presentations of traumatic brain injury delirium have a better physical and cognitive prognosis than hypoactive presentation.12 Hyperactive delirious medical patients (alcoholics excluded) had a high rate of full recovery, whereas mortality was highest in the mixed subtype.13 If such motoric subtypes have distinctive symptom patterns, these may reflect separate pathophysiological processes and may have differing responsivities to the range of somatic interventions that are becoming increasingly available for the management of this complex condition. Thus far, only alcohol withdrawal delirium is associated with a different EEG and SPECT scan presentation than other forms of delirium.14
In this study, we investigated the relationship between motoric subtypes and delirium symptoms in order to better understand delirium phenomenology. We used standardized delirium symptom ratings in conjunction with a previously published definition of three motoric subtypes to describe a general hospital consecutive sample of consultation-liaison referral patients.
METHODS
Subjects
The patients included in this study have been described previously in a report about the relationship between delirium subtypes and ward management practices.11 These 46 patients were consecutive referrals over a 4-month period from general medical wards to a psychiatric consultation service in a large teaching hospital, the Mater Hospital in Dublin. All patients met ICD-10 criteria for delirium.4 Demographic details of patients were recorded at the time of consultation.
Procedures
Each patient was assessed on the first evaluation day by an attending psychiatrist (D.J.M., D.O'H., E.O'M.) using the Delirium Rating Scale (DRS), a 10-item clinician-rated scale that rates the severity of a broad constellation of delirium symptoms and generates a total severity score for delirium.6 It has the specific advantage of not being heavily reliant on cognitive impairment and thus allows the assessment of the interrelationships among a wide range of delirium symptoms. It has good sensitivity, specificity, and interrater reliability.15,16 The three DRS raters underwent a number of practice ratings together prior to the study.
Patients were later subdivided into delirium subtypes by group consensus, using all available data from a review of case records and the information from the consultation. The DRS ratings were not used for the purpose of motoric subtyping. Clinical information to determine subtypes was gathered over the course of several visits during both daytime and nighttime. The phenomenological subtypes used were initially described by Liptzin and Levkoff (Table 1).9 Patients were divided into hyperactive, hypoactive, and mixed subtypes according to the presence or absence of defined symptoms. There is little overlap between these symptoms and the DRS; the latter has only one item for psychomotor activity, and that item does not distinguish hyperactive from hypoactive states. Since these delirium groups are primarily defined according to motor activity levels, they will be referred to as motoric subtypes.
The referring doctor was requested to indicate the noticed onset of delirium symptoms, the most likely primary etiology of the delirium, and whether there was any clinical evidence of underlying dementia (“acute on chronic picture”). Duration of delirium episodes was determined at the time of consultation, on the basis of the referring physician's estimate of the date of symptom onset.
Statistical Analyses
Data analysis was conducted by using the Statistical Package for the Social Sciences for Windows (6.1.2). Demographic data are expressed in means and standard deviations. Comparisons of age, duration of symptoms, and total DRS scores between motoric subtypes were made by using one-way analysis of variance (ANOVA) and least significant difference (LSD) test of the level of significant difference. DRS items were compared between motoric subtypes by using the Kruskal-Wallis one-way ANOVA test, a generalization of the Wilcoxon rank sums test. A significance level of P<0.05 was used, except for analyses of DRS item scores between the motoric subtypes, which were compared by using a cutoff value of P≤0.005 to correct for multiple comparisons of these items. Pearson product-moment correlation coefficient was calculated for the relationship between age and total DRS score.
RESULTS
Demographics
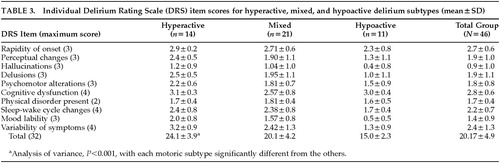
Mean age was 60.1±19.5 years (range 21–89), and 26 patients (56%) were female. Mean total DRS score was 20.17±5.0 points (range 13–30). An inverse correlation was noted between age and severity of delirium (r=–0.54; P<0.001). Assignment of motoric subtypes revealed that 11 (24%) were hypoactive, 14 (30%) were hyperactive, and 21 (46%) were mixed. The motoric subtypes were then compared with each other in relation to age, gender, total DRS scores, and duration of symptoms (Table 2). At the time of the assessment, hyperactive subtype patients had experienced a longer duration of symptoms than either the mixed or hypoactive groups (F=7.92, df=43, P<0.01). All three groups differed from one another with regard to total DRS scores such that the hyperactive group had the highest score, the hypoactive group had the lowest, and the mixed group was intermediate (F=17.79, df=43, P<0.001; Table 3). There were no significant age or gender differences among the motoric subtypes.
The most likely primary etiologies for delirium are represented in Table 4. Six cases had a history consistent with delirium superimposed on underlying dementia. The mean age of this demented group was 74.8 years and mean DRS score was 17.66±3.71. Of these 6 cases, 3 were hypoactive, 1 hyperactive, and 2 mixed motoric subtypes.
DRS Items and Motoric Subtypes
The relationship between motoric subtype and specific DRS items is shown in Table 3. DRS items, except for cognitive status and physical disorder, showed the same pattern of relationships among motoric subtype as did total DRS scores, either when compared by mean values or ranked by the Kruskal-Wallis test.
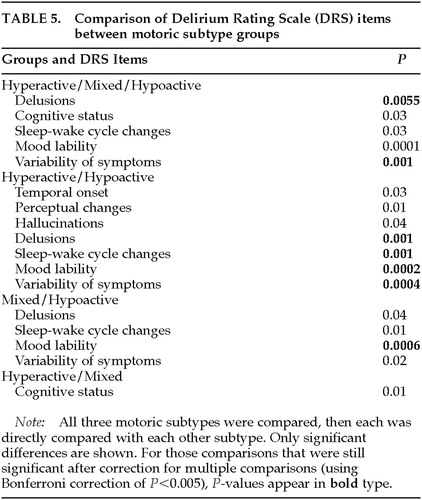
Table 5 lists the DRS items that were significantly different among the three groups and for pairwise comparisons. The greatest number of differences was found between hyperactive and hypoactive groups, with 7 out of 10 items being different. After Bonferroni correction, however, only delusions, sleep-wake cycle disruption, mood lability, and variability of symptoms distinguished the hyper- and hypoactive groups. Three of these same four items (all except sleep-wake cycle) distinguished all three motoric subtypes from each other. No individual item distinguished mixed from hyperactive subtypes. Mixed subtype differed from hypoactive subtype only on mood lability. Interestingly, the three groups did not differ on cognitive status.
DISCUSSION
We have described 46 consecutively referred delirium patients who met ICD-10 criteria, with the aim of studying the relationship between specific delirium symptoms and motoric subtypes that were independently assessed. We used the DRS to rate severity of individual symptoms and compared these among three groups (hyperactive, hypoactive, and mixed) distinguished by their psychomotor presentations according to criteria first delineated by Liptzin and Levkoff.9 The DRS, unlike the subtype criteria, does not make a priori assumptions about which behavior or motoric symptoms might differentiate hyper- from hypoactive presentations of delirium. Furthermore, the Liptzin and Levkoff subtype classification includes many symptoms that are not rated in the DRS, although two DRS items (psychomotor behavior and mood lability) would be likely to overlap. In addition, the DRS psychomotor item does not distinguish hypo- from hyperactive cases, instead rating only the severity of either presentation. Using these assessment tools, we found that several delirium symptoms significantly distinguished the motoric subtypes. Our findings are consistent with a previous study of phenomenologic subtypes of delirium17 that used different measurement tools. Our findings provide support for the suggestion that the syndrome of delirium is composed of a collection of separate components or symptoms, the expression of which might vary according to different etiologies.
Overall DRS scores significantly distinguished the motoric subtypes, with mean scores for the hyperactive subtype more than 9 points greater than for the hypoactive subtype. The mixed subtype was intermediate between these extremes, and, surprisingly, no item score distinguished this group from hyperactive cases, even though the mixed group's total DRS score was significantly lower. The symptoms that contributed to the differences among the three groups were delusions, mood lability, and variability of symptoms. Hypoactive and hyperactive groups also differed on sleep-wake cycle disturbances. Interestingly, items with similar scores among the three groups included temporal onset, cognitive impairment, physical disorder, and sleep-wake cycle disturbance. Although also not significantly different, scores for psychomotor behavior, hallucinations, and perceptual disturbances were highest for the hyperactive group and lowest for the hypoactive group.
In findings similar to ours, Ross et al.17 found no difference in cognitive functioning, using the Mini-Mental State Examination, between hyperactive and hypoactive subtypes. However, they did find that agitated behavior, psychosis, mood lability, rapid speech, and incoherence were more common in hyperactives. Similarly, Koponen et al.18 found no differences in EEG spectral analysis or cognitive function between hyper- and hypoactive patients.
Lipowski,19 however, described a third, “mixed,” group that Ross et al.17 did not report. Subsequent studies suggest it may in fact be the most common form of the syndrome.9,11,18 It has been suggested that this mixed category is simply a reflection of the inherent variability of delirium and the tendency of many patients to fluctuate in their symptom severity—although any symptom (mood lability, psychosis, cognition) can fluctuate, not just psychomotor behavior. It is possible that those patients who fall into this mixed group have endured a longer duration of delirium at the time of assessment, with more opportunity for multiple causes to contribute to symptom profile. However, Rudberg et al.20 assessed the pattern of delirium symptoms occurring in hospitalized elderly patients, using daily DRS ratings, and found that there were no patterns of change over time in specific DRS items. When Liptzin and Levkoff9 studied these subtypes in relation to delirium course and outcome, they found that patients of the mixed subtype had longer hospital stays and higher 6-month mortality rates than the other groups.
Our hyperactive group had a longer duration of recognized delirium at the point of referral than the other motoric subtypes. However, previous work has suggested that hyperactive patients are recognized and referred earlier because of the management problems that their behaviors present and that less active patients are more likely to go unnoticed or be misdiagnosed as depressed.21 This discrepancy may be related to the higher rate of intervention with psychotropic medications that is associated with hyperactive presentation of delirium.11,22 The relative delay in consultation in hyperactive cases may be due to an initial misdiagnosis of psychosis, or it may reflect an observation period after the commencement of pharmacologic intervention by the primary team. It may also be a reflection of the broad range of symptoms used in addition to motor activity to define the motoric subtypes in our study—which in hyperactive patients would include behavioral symptoms that get the attention of caregivers. A study of consecutively admitted patients who are at risk of delirium, and not a referral sample, could address this methodological problem. In view of evidence indicating differing management practices between motoric subtypes of delirium,11 further work is needed to investigate to what extent the superior outcome noted in patients of hyperactive motoric profile is related to earlier recognition, more active management, or underlying pathophysiology.
The relationship between symptoms or subtypes of delirium and underlying etiology is an uninvestigated area, except for substance-related deliria. Recent work suggests that, for example, delirium due to drug withdrawal is more likely to be of the hyperalert-hyperactive type and metabolic encephalopathy is more likely to be of the hypoactive-hypoalert type.17 In addition, other work has suggested that different delirium subtypes may be associated with particular disruption of the activity of specific neurotransmitter systems.23 For example, anticholinergic intoxication states and delirium from traumatic brain injury may be more often hyperactive. Delirium from traumatic brain injury, stroke, hypoxia, thiamin deficiency, and hypoglycemia may be due to reduced CNS cholinergic activity,14 but motoric subtype has not been systematically studied in these etiologic groups. Other evidence suggests that sedative-hypnotic and alcohol-related causes of delirium are associated with disturbances of GABAergic activity, at least in part.14
If different etiologies for delirium have distinctive symptom profiles, these may reflect differing pathophysiological patterns. The relationship between etiology and motoric subtype warrants a well-designed prospective study. Such study may provide important insights into crucial aspects of delirium management, including differing responsiveness to the increasing range of therapeutic modalities available for the treatment of delirium.
Although these data provide support for a number of important relationships between phenomenology and motoric subtype of delirium, the study has a number of limitations. The findings relate to referrals to a general hospital consultation-liaison service and thus may not be generalizable to other populations. The sampling methods may have led to an overrepresentation of certain delirium subtypes or particular problems encountered in delirious patients. Referral samples also make the assessment of symptom duration more difficult. Our group included 6 cases with a suspected underlying dementia that may have altered the presentation of delirium.14 However, in a recent factor-analysis comparison of delirious and delirious-demented elderly patients, it appeared that delirium phenomenology largely overshadowed dementia symptoms, although the groups were not completely alike.24 The DRS has many advantages for application in studies of this type, but it does not specifically include an item related to attention levels, which are considered by some researchers to be a fundamental feature of delirium, nor does it separate motoric subtypes into separately rated items. The use of standardized cognitive tests in addition to the DRS might have elucidated more subtle differences in severity or type of cognitive impairments among motoric subtypes. Future studies of motoric subtypes should include 24-hour objective motor activity monitoring in addition to clinical ratings.
ACKNOWLEDGMENTS
Presented at the 11th annual meeting of the American Association for Geriatric Psychiatry, San Diego, CA, March 10, 1998.
 |
 |
 |
 |
 |
1 Thomas RI, Cameron DJ, Fahs MC: A prospective study of delirium and prolonged hospital stay. Arch Gen Psychiatry 1988; 45:937–940Crossref, Medline, Google Scholar
2 Rabins PV, Folstein MF: Delirium and dementia: diagnostic criteria and fatality rates. Br J Psychiatry 1982; 140:149–153Crossref, Medline, Google Scholar
3 Francis J: A half-century of delirium research: time to close the gap. J Am Geriatr Soc 1995; 43:585–586Crossref, Medline, Google Scholar
4 World Health Organization: The Tenth Revision of International Classification of Diseases and Related Health Problems (ICD-10). Geneva, World Health Organization, 1992Google Scholar
5 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994Google Scholar
6 Trzepacz PT, Baker RW, Greenhouse J: A symptom rating scale for delirium. Psychiatry Res 1988; 23:89–97Crossref, Medline, Google Scholar
7 Albert MS, Levkoff SE, Reilly C, et al: The Delirium Symptom Interview: an interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol 1992; 5:14–21Crossref, Medline, Google Scholar
8 Wada Y, Yamaguchi N: Delirium in the elderly: relationship of clinical symptoms to outcome. Dementia 1993; 4:113–116Medline, Google Scholar
9 Liptzin B, Levkoff SE: An empirical study of delirium subtypes. Br J Psychiatry 1992; 161:843–845Crossref, Medline, Google Scholar
10 Olofsson SA, Weitzner MA, Valentine AD, et al: A retrospective study of the psychiatric management and outcome of delirium in the cancer patient. Support Care Cancer 1996; 4:351–357Crossref, Medline, Google Scholar
11 Meagher DJ, O'Hanlon D, O'Mahony E, et al: A study of environmental strategies in the study of delirium. Br J Psychiatry 1996; 168:512–515Crossref, Medline, Google Scholar
12 Reyes RL, Bhattacharyya AK, Heller D: Traumatic head injury: restlessness and agitation as prognosticators of physical and psychological improvement in patients. Arch Phys Med Rehabil 1981; 62:20–23Medline, Google Scholar
13 Kabayashi K, Takeuchi O, Suzuki M, et al: A retrospective study on delirium subtype. Jpn J Psychiatry Neurol 1992; 46:911–917Medline, Google Scholar
14 Trzepacz PT: The neuropathogenesis of delirium: a need to focus our research. Psychosomatics 1994; 35:374–391Crossref, Medline, Google Scholar
15 Trzepacz PT: A review of delirium assessment instruments. Gen Hosp Psychiatry 1994; 16:397–405Crossref, Medline, Google Scholar
16 Trzepacz PT: The Delirium Rating Scale: its use in consultation/liaison psychiatry research. Psychosomatics 1999; 40:193–204Crossref, Medline, Google Scholar
17 Ross CA, Peyser CE, Shapiro I, et al: Delirium: phenomenologic and etiologic subtypes. Int Psychogeriatr 1991; 3:135–147Crossref, Medline, Google Scholar
18 Koponen H, Partanen J, Paakkonen A, et al: EEG spectral analysis in delirium. J Neurol Neurosurg Psychiatry 1989; 52:980–985Crossref, Medline, Google Scholar
19 Lipowski ZJ: Delirium: Acute Confusional States. New York, Oxford University Press, 1990Google Scholar
20 Rudberg MA, Pompei P, Foreman M, et al: The natural history of delirium in older hospitalized patients: a syndrome of heterogeneity. Age Ageing 1997; 26:169–175Crossref, Medline, Google Scholar
21 Nicholas LM, Lindsey BA: Delirium presenting with symptoms of depression. Psychosomatics 1995; 36:471–479Crossref, Medline, Google Scholar
22 Fainsinger RL, Tapper M, Bruera E: A perspective on the management of delirium in terminally ill patients on a palliative care unit. J Palliat Care 1993; 9:4–8Medline, Google Scholar
23 Trzepacz PT: Anticholinergic model for delirium. Seminars in Clinical Neuropsychiatry 1996; 1:294–303Medline, Google Scholar
24 Trzepacz PT, Mulsant BH, Dew MA, et al: Is delirium different when it occurs in dementia? A study using the Delirium Rating Scale. J Neuropsychiatry Clin Neurosci 1998; 10:199–204Link, Google Scholar



