The Spectrum of Organic Depersonalization
Abstract
Depersonalization and derealization are commonly reported in the general population as a response to stress. The symptoms have also been described in patients with a primary psychiatric or organic diagnosis, where their secondary status precludes a DSM-IV diagnosis of depersonalization disorder. The authors present 4 new cases of depersonalization in patients with an underlying organic condition, along with 47 cases from the literature in which the available information permits diagnosis of organic depersonalization. Information from case series documenting depersonalization in the context of medical illnesses is also presented and the underlying etiology discussed. Epilepsy and migraine appear to be the disorders most commonly associated with depersonalization. Left-sided temporal lobe dysfunction and anxiety are suggested as factors in the development of depersonalization; however, further studies are needed to determine the relationship. The introduction to the DSM-IV of an organic subtype of depersonalization disorder would facilitate research in this area.
The symptom of depersonalization (DP) has been defined in DSM-IV as an experience of feeling detached from and as if an outside observer of one's mental processes or body, while maintaining intact reality testing; derealization (DR) has been defined as the sensation that the external world is strange or unreal.1 Although these symptoms are classified separately under dissociative disorders in the DSM-IV, they often coexist,2 and both consist of altered perceptions of the self and the environment.1 DP and DR are commonly reported in the general population3–5 and in patients with a variety of psychiatric disorders.6–11 The symptoms tend to be transient and of short duration; however, they may persist and develop into the syndrome of depersonalization disorder, which can be diagnosed when persistent or recurrent episodes of DP cause distress and occur in the presence of intact reality testing.1 The diagnostic criteria are similar in ICD-10; however, in this system, derealization is included along with DP,12 whereas it is classified separately as a variant of “dissociative disorder not otherwise specified” in DSM-IV.1 The syndrome tends to begin in adolescence and characteristically has a chronic course, although the intensity of the symptoms may vary. According to the diagnostic criteria, however, the disorder must not occur exclusively during the course of another mental disorder and must not be due to the effects of a drug (prescribed or illicit). Furthermore, a preexisting diagnosis of an organic disorder precludes the diagnosis.1,12 However, the validity of this construct has been argued.13
This article reviews the English-language literature documenting depersonalization and/or derealization in patients with an underlying organic condition (not attributable to substance abuse or intoxication) and presents four new cases of organic depersonalization. The relevance of the concept of organic depersonalization for determining the underlying mechanism of its development and for understanding its neural correlates is discussed.
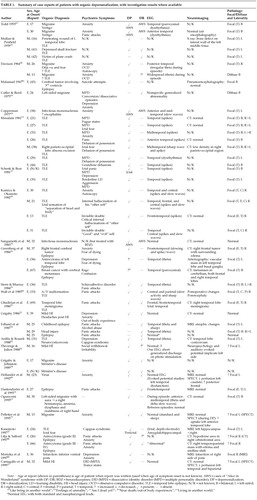
METHODS
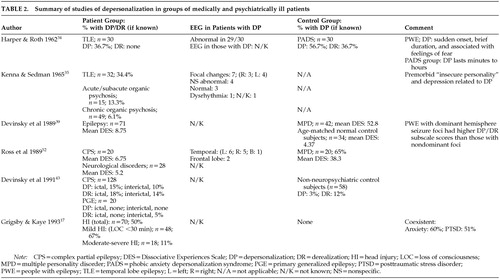
A computerized literature search was performed (using the PsychLit and MEDLINE databases), employing the following terms: depersonalization, derealization, dissociation, multiple personality disorder, autoscopy, and “Alice in Wonderland” syndrome, combined with the following: organic, neurological, epilepsy, migraine, cerebral tumors, delirium, encephalitis, head injury, and traumatic brain injury. In addition, articles reviewing the psychiatric and neuropsychiatric comorbidity of organic disorders were searched for cases of DP. Similarly, depersonalization case series were searched for patients with a coexistent organic disorder. Reports of acute stress and cases of posttraumatic stress disorder were also reviewed and included if symptoms of DP/DR occurred in the context of an underlying organic disorder. Articles were included only if there were reasonable grounds given for a diagnosis of DP. These methods resulted in over 60 case reports and series, from which enough information was documented in 47 cases to merit detailed tabulation. Case reports are summarized in Table 1 and studies in Table 2.
LITERATURE REVIEW
It has long been thought that DP may have an organic etiology. Mayer-Gross14 regarded it as resulting from a “preformed functional response of the brain,” and Ackner15 as “the result of a cerebral dysfunction, which itself is specific, but which may be set in motion by a number of different causes.” Ackner15 asserted that DP occurs in a variety of conditions, including epilepsy, head injury, encephalitis, tumor, chorea, intoxication, carbon monoxide poisoning, and toxic and delirious states. Despite this extensive list, most subsequent studies have focused on the first two conditions.
It is difficult to determine with certainty that depersonalization is occurring secondary to an organic disorder rather than a psychiatric disease, because in most medical and neurological conditions, psychiatric disorders coexist. In fact, Ackner himself maintained that “depersonalization symptoms can never be entirely organic in origin for, being concerned with changes in experience, they are indivisibly related to the mental functioning of the individual…their occurrence will be related to periods of emotional stress which make manifest a latent deficiency in the biological substrate for integration.”15 Cohen16 linked these two concepts with the hypothesis that all depersonalization and derealization was “organic” in nature, the result of the changes in metabolism and cerebral blood flow produced by hyperventilation.
RESULTS
Traumatic Brain Injury (TBI)
Depersonalization commonly accompanies acute stress9,17–19 and is thought to play an important role as a psychological defense mechanism protecting against the long-term sequelae of trauma. Thus, it is difficult to determine whether depersonalization following TBI is secondary to the physical or the psychological effects of the injury. Additional complications include evaluating the role of comorbid psychiatric problems such as depression, anxiety, and posttraumatic stress disorder (PTSD).
The literature on the development of depersonalization following TBI is surprisingly sparse and mainly consists of single case reports. Thivierge and Julien20 described a patient who developed headaches, blurred vision, and depersonalization following a minor head injury with no or minimal loss of consciousness and no reported posttraumatic amnesia (PTA). All neurological investigations were normal except for a marked asymmetry of the late cortical auditory evoked response, which persisted for 2 years and then normalized within months of clinical improvement. Recently, Cantagallo et al.21 described the case of a 32-year-old man who developed transient episodes of DP (as well as one episode of “multiple personality”) 6 weeks following a mild brain injury (PTA <1 hour). He had not previously suffered episodes of dissociation, and episodes of DP ceased within 1 year of the accident. The CT scan was normal, but left temporal and biparietal hypoperfusion was visible on a [99mTc]HMPAO SPECT scan. Grigsby22 reported a case of a patient who developed DP (an “out-of-body” experience) at the scene of a car accident but then went on to suffer from both DP and DR for several months following the mild closed head injury.
Subsequently, Grigsby and Kaye17 assessed 70 patients who had been referred for neuropsychological assessment on average 22.3 months following a head injury (Table 2). Half reported feelings of unreality following the head injury, mostly occurring at the time of the injury. Patients with the most minor head injuries (no loss of consciousness or unconsciousness <30 min) were more likely to develop DP/DR (67%, compared with 11% of those with loss of consciousness ≥30 min). Only 6% experienced an isolated episode of unreality at the time of the injury, whereas the majority described frequent recurring and remitting episodes of DP/DR. There was no association with any neuropsychological or personality measures or with ongoing litigation. Their sample was atypical, however, in that although most head injuries occur in men, their sample contained more women. Furthermore, most patients do not require referral for neuropsychological assessment following TBI, with an estimate of only 20% continuing to have problems 6 weeks after an injury.23 Grigsby and Kaye17 extrapolated from these figures and estimated that a minimum of 13% of patients would develop depersonalization following a head injury. Half of the patients with symptoms of DP met DSM-III-R criteria for PTSD, and in fact only 5 patients with PTSD did not also experience DP. Thus, it could be argued that DP in patients with mild TBI was merely an expression of a psychogenic disorder.
Epilepsy
The association between DP and epilepsy has long been reported. Jackson and Colman in 189824 described the “dreamy state” in a patient with temporal lobe epilepsy (TLE) secondary to a lesion in the left uncinate gyrus. However, it is not always clear from the published literature whether the DP is occurring interictally or as part of the seizure. In patients with frequent seizures, this differentiation may be difficult without video-EEG telemetry. Furthermore, the patients often had other comorbidity, whether organic (previous head injury, migraine) or psychiatric (concomitant depression, anxiety, substance misuse). Thus it is difficult to determine the relationship between DP and epilepsy. For example, Langs et al.25 reported a case of a man who developed episodes of derealization the day after alcohol and cannabis ingestion. However, he was also sleep-deprived and had experienced a generalized tonic-clonic seizure 15 minutes after the onset of the DR (which thus may have been prodromal). Following the index episode he experienced further episodes of DR along with a fear of having another seizure (whether or not he had ingested cannabis). Determining whether it was the alcohol, cannabis, anxiety, or seizure activity that was responsible for the episodes of DR is impossible.
Case Reports:
Greenberg et al.26 described 4 patients suffering from complex partial seizures secondary to malignant disease. Three developed episodes of an altered perception of reality accompanied by fear, which they interpreted as indicating that they had died or were about to die. Anticonvulsant medication appeared to terminate these experiences, which were believed to be ictal or para-ictal in origin.
One single case report by Kanemoto27 describes peri-ictal depersonalization. Following clusters of simple partial seizures experienced as ictal fear, the patient developed episodes, lasting up to several weeks, of the Capgras syndrome (the delusion that a person close to the patient had been replaced by an imposter) along with depersonalization. Concurrent depth electrode EEG recordings revealed epileptiform discharges originating from the left amygdalohippocampal region.
Davison28 described episodic depersonalization in 3 patients who had been diagnosed as having temporal lobe epilepsy; however, the EEG during attacks did not clearly demonstrate ictal activity. In one patient, who also suffered from migraine, the DP lasted for up to two hours and was accompanied by a sensation of fear. It ended abruptly and was followed by exhaustion. The EEG during the episode revealed irregular theta rhythm over the right posterior temporal region. Another patient, who also suffered from migraine, experienced an unpleasant smell for approximately five minutes during the episodes of DP. During an episode, EEG recording revealed bilateral theta activity during overbreathing. In the final case, the episodes followed consumption of alcohol. Although they were reported as being modified by the anticonvulsant phenytoin, there is little evidence to support a diagnosis of epilepsy; therefore only the first two cases are included in Table 1.
Several other case reports have documented DP and DR along with symptoms of panic, which were relieved by anticonvulsant medication.29–31 EEG studies revealed temporal lobe29,30 or frontotemporal abnormalities.31
Case Series:
Most series have found symptoms of DP to be more commonly associated with TLE than other forms of epilepsy.32 Mullan and Penfield33 reported ictal phenomena in patients undergoing presurgical evaluation for TLE. Ictal feelings of unreality were described in 3 patients with epilepsy originating from the left temporal lobe, which in all cases was dominant for speech. Depersonalization, but not derealization, was reported in 36.7% of a series of patients with TLE.34 The DP tended to be of abrupt onset and short duration and was often accompanied by a sensation of fear.
Kenna and Sedman35 reported DP in 11 of 32 patients with epilepsy who had been referred to the psychiatric services. These 11 patients suffered from psychomotor or multiple types of seizure, were predominantly female, and tended to be slightly older than patients without DP. No clear laterality effects were noted on routine EEG studies. There was, however, an association of DP with current depression and an “insecure” personality.
Smirnov36 described the ictal experiences in 39 patients with temporal lobe tumors. In patients with right-sided lesions, the main symptoms were the emotions of fear or grief, usually accompanied by viscero-autonomic disturbances, along with both derealization and depersonalization. Also experienced were olfactory and auditory hallucinations, déjà vu, and jamais vu. Patients with left-sided tumors tended to experience anxiety accompanied by speech disturbances, along with auditory hallucinations, automatisms, and compulsive thoughts and reminiscences.
Schenk and Bear37 reported recurrent dissociative experiences in one-third of their patients with TLE, mostly occurring in the female patients. The episodes always followed the onset of seizures, usually by months to years. The authors presented 7 cases of multiple personality disorder (MPD) in patients with TLE, 2 of whom also experienced episodic depersonalization. The authors were confident that these episodes occurred interictally.
Several studies have documented the frequency with which symptoms of depersonalization occur in patients with epilepsy and compared this with experiences encountered in the general and psychiatric populations. Silberman et al.38 showed that patients with epilepsy (PWE) suffering from complex partial seizures, as well as patients with affective disorders, had more experiential phenomena than control subjects and tended to experience them more during episodes of illness. Derealization was reported by 16% of PWE, 18% of patients with affective disorders, and 3% of control subjects. Other dissociative symptoms reported by patients, but by none of the control subjects, included altered body size (11% of PWE, 9% of patients with affective disorders); body part dissociation (19% of PWE, 7% of affective-disorder patients); and autoscopic states, which were experienced more by patients with affective disorders (14%) than PWE (5%). Interestingly, there were no group differences for “depersonalization,” which was experienced by 19% of PWE, 14% of patients with affective disorders, and 10% of control subjects.
Devinsky et al.39 compared scores on the Dissociative Experiences Scale (DES)40 between normal control subjects (n=34), patients with multiple personality disorder (n=42), and 71 PWE (12 with generalized and 59 with complex partial seizures). A cutoff score of 15–20 on the DES has been thought to detect dissociative disorders.41 The DES has three subscales: amnesia/dissociation, absorption/imaginative involvement, and depersonalization/derealization.42 Devinsky et al.39 found that PWE had DES scores between those of MPD patients and normal subjects. Patients with partial seizures had higher scores than those with generalized seizures on the dissociation subscale only. Furthermore, patients with dominant hemisphere foci had higher DP subscale scores than those with nondominant foci. Overall, they found that one-fifth of PWE had significant dissociative experiences.
They also reported the findings of resting EEGs and prolonged video-EEG telemetry on 6 of these patients during dissociative episodes. All were originally suspected of having a diagnosis of epilepsy, but the telemetry refuted this and all were found to suffer from MPD with depersonalization. The EEGs both between and during episodes were abnormal in 5 of the patients; however, the features were nonspecific variants such as intermittent temporal theta activity or occasional spikes, with no clear epileptiform activity during the episodes.
In a later study, Devinsky and co-workers43 compared the experience of dissociative symptoms in patients with focal and primary generalized epilepsy and non-neuropsychiatric control subjects. Ictal depersonalization was reported by 15% of patients with partial seizures, occurring interictally in 10%. This symptom was reported by 3% of control subjects but by none of the patients with primary generalized epilepsy. Derealization was more common: it occurred ictally in 18% with complex partial seizures, and interictally in 14% with partial seizures, 5% with primary generalized epilepsy, and 12% of control subjects. Related symptoms such as distortion of size/shape and distance were not experienced by the control group, but occurred interictally in both groups with epilepsy and also ictally in those with partial seizures.
The same group44 administered the DES to 169 PWE and 132 patients with conversion nonepileptic seizures (NES). The mean DES score for PWE was 12.7, and the investigators commented that this might be elevated because of items that may reflect memory and attention. Patients suffering NES had a mean DES score of 15.1 and scored significantly higher than PWE on the DP/DR subscale. Patients who had histories of childhood physical or sexual abuse scored highest on the absorption-imaginative involvement subscale regardless of the origin of their seizures.
A recent study by Kuyk et al.45 assessed psychological and somatoform dissociation in 94 patients with TLE, 40 with extratemporal and generalized epilepsy, and 65 with NES. They found higher psychological dissociation in patients with TLE and NES compared with nonclinical control subjects. Furthermore, patients with NES also showed somatoform dissociation compared with the other groups.
Persinger and Makarec46 compared patients suffering from PTSD, anxiety-depersonalization, or complex partial seizures with a control group. All of the patient groups had elevated depersonalization scores, the highest being in those with epilepsy, and the authors concluded that such symptoms should be viewed as occurring along a continuum from “normal” individuals to people with epilepsy.
Toni et al.,47 using a semistructured interview, found features of depersonalization and derealization in 61% of a sample of 41 patients with complex partial seizures. The symptoms were similar to those experienced by patients with phobic-anxiety depersonalization, comprising mainly feelings of detachment from the environment (DR), feelings that the external world is unfamiliar (DR), and feelings of losing self-control (DP).
Autoscopy (or heautoscopy), the visual experience of seeing an image of oneself in external space viewed from within one's body,48 and out-of-body experiences, in which there is a sensation of leaving one's body and viewing the image from outside, are associated with dissociation and depersonalization. A meta-analysis in 199448 of 56 published cases of autoscopy revealed that 59% of the patients had a neurological illness and 32% had epilepsy (predominantly TLE). There were no significant laterality effects; however, in cases with focal pathology, the images tended to appear in the contralateral field. DP occurred in 18% of the 56 cases, the commonest coexisting psychiatric disorders being depression, anxiety, and panic. Kamiya and Okamoto49 described 9 cases of “double consciousness” in patients with epilepsy. Three had episodes of autoscopy, 2 had a sensation of an invisible double outside their body (often referred to as Doppelgänger), and 4 had a sensation of a double identity (often good versus bad) inside their body. Four (all with right-sided EEG abnormalities) also experienced depersonalization, and these tended to belong to the latter group (invisible double identity within the body).
A similar phenomenon is that of “multiple personality disorder” (MPD)—now referred to as dissociative identity disorder (DID)—which is defined as a dissociative disorder characterized by the existence of two or more distinct personal identities within a single individual, which recurrently take control of the person's behavior.1 Depersonalization has been reported in 38% to 65% of patients with MPD.50–52
MPD was first described in a patient with epilepsy in 1898.24 Since then, several studies have explored this relationship. Mesulam53 noted MPD in 7 of 61 patients with TLE seen in a behavioral neurology unit over a period of 1 year. In 4 patients, symptoms of depersonalization were also present. He also described a further 3 cases of patients with epilepsy, depersonalization, and a delusion of possession (by evil or God). In all cases, the EEG was abnormal (Table 1. Benson et al.54 reported 2 PWE with dual personality and Capgras syndrome who shifted between personalities on recovery from seizures. However, the accuracy of diagnosis of MPD in the latter two studies has been questioned.52 Drake55 also described 5 patients who exhibited different personalities while in the postictal state. Attempts have been made to compare EEGs recorded during different personalities in an individual patient, but in most cases changes have been confined to those associated with alteration in concentration and mood.56,57 Coons et al.51 found a 10% incidence of epilepsy in 50 patients with MPD, and abnormal EEGs (not associated with medication) in 14% (usually mild, nonspecific slowing, but also spikes affecting the frontal, temporal, and parasagittal areas). Ross et al.,52 however, did not find cases of MPD or of raised DES scores in a series of 30 PWE.
Stimulation Studies:
The “dream-like” state has been elicited by electrically stimulating the medial temporal lobe in patients undergoing assessment for epilepsy surgery.58–60 However, the specific anatomical site or even the hemisphere that would habitually produce particular experiential sensations when stimulated has not been identified. Gloor et al.60 concluded that it was the personal characteristics and memory bank of the patient that were the major factors in determining the mental phenomena evoked.
Migraine
Shorvon et al.61 found that 38% of patients with DP also suffered from migraine. Since this early study, several others have documented this association. (Table 1 and Table 2).9,28,34,62 Ogunyemi63 performed EEG studies in a patient at the time he was suffering from a prolonged (>1 hour) migrainous aura accompanied by depersonalization, “as if he was outside his body.” His EEG showed intermittent, asynchronous, focal theta and delta slow waves in the anterior-midtemporal regions bilaterally, which resolved when he was symptom free. Derealization accompanying MPD has also been reported in a patient with migraine.64 Furthermore, headaches were commonly reported when patients with MPD were assessed by clinicians,50 and in one study, 26% of patients described their headaches occurring either just before or during the transition from one personality to another.51
Other experiences related to DP and DR, such as autoscopy, have also been described accompanying migraine.65,66 The patients experienced a sensation of being two people—the secondary body being the more real, thinking, feeling and controlling all movements, the original body being devoid of feelings. The sensation lasted only seconds but tended to occur either as the migraine aura, with the headache, or immediately afterwards. In some cases, the autoscopy preceded the onset of migrainous headaches by several years. Todd67 reported 5 patients (4 female) who suffered from migraine or migraine equivalent (migraine symptoms such as nausea, giddiness but without headache) and had recurrent episodes (occurring over many years, in some cases), which he described as the syndrome of “Alice in Wonderland” (AWS). The patients described objects or their own body (or isolated parts) as changing in size. These experiences were accompanied by transient depersonalization in 2 cases. In 2 patients, the attacks were accompanied by an alteration in the perception of time, and in 2 other patients autoscopy occurred (again with the sensation that the secondary body contained the mind). All of the patients also suffered episodes of vertigo or giddiness, and all had a psychiatric comorbidity—predominantly of anxiety.
Since Todd's series was published, further, similar cases of AWS have been reported in both adults and children, either associated with migraine62,68 or infections.69–74 These case reports of body image distortion, often in association with depersonalization and/or derealization, suggest parietal lobe pathology. However, the frequent accompanying symptoms of fear,62 anxiety, and panic implicate the temporal lobe. In most cases, neuroimaging and EEG studies were normal; however, nonspecific findings have been reported, including temporal lobe dysrhythmia,67 left anterior and midtemporal slow waves,69 and parieto-occipital sharp waves.72 [99mTc]HMPAO SPECT brain imaging in a patient with migraine and DP demonstrated an increase in uptake in the left anterior temporal lobe when symptomatic. Between episodes, there was a decreased uptake in the left temporal lobe.75
Vertigo
DP and DR have been reported in patients suffering from vertigo,39,67,76,77 either due to Ménière's disease78 or following head injury.17 Fewtrell and O'Connor79 reviewed the association between depersonalization and dizziness per se. They concluded that either these were identical experiences described in different ways or they were distinct experiences lying on opposite ends of a continuum of a disturbance in “self–world” relations.
Cerebral Tumors and Cerebrovascular Disease
Lilja and Salford80 compared the presenting symptoms in patients with high-grade and low-grade gliomas. Panic attacks with prominent experiences of DP and DR tended to occur early in the course of low-grade frontal lobe tumors. Epilepsy tended to occur more frequently in patients with low-grade tumors. Several single case reports also document DP in patients with cerebral tumors (Table 1); however, in all cases, concomitant epilepsy81,82 and psychiatric symptoms83 complicate evaluating the etiological significance of the tumor in the development of DP.
Similarly, DP has been reported in patients with cerebrovascular disease, again in association with panic84 and depression.85 (Table 1).
NEW CASE HISTORIES
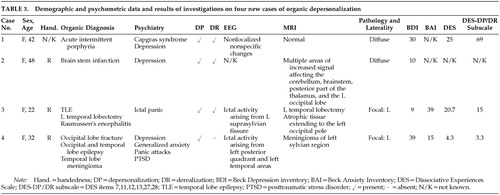
Demographic and psychometric data and investigative results on four new cases seen by the authors are summarized in Table 3.
Case 1. A 42-year-old woman with no personal or family history of any neuropsychiatric disorder was diagnosed with acute intermittent porphyria at the age of 39. Her attacks tended to occur monthly, generally premenstrually, and consisted of intense abdominal pain radiating to both legs, accompanied by headache, nausea, and occasionally vomiting. During severe attacks she would become mute, later stating that she tried to talk but “the words would not come out.” Following a severe episode, she became comatose for 2 weeks and on regaining consciousness, was found to be delirious. After 1 month, the delirium cleared, revealing a Capgras syndrome with reduplicative paramnesia. Although she could recognize her husband, her children, and her house, she believed that there was “something different about them.” She thought that either everything had been duplicated or that she had been “taken by aliens to another planet where things were similar.” She also experienced intense symptoms of depersonalization. She stated that her body felt strange, as though it did not belong to her, and that she did not know whether she existed or not. She also felt that she was not in control of her behavior. She believed that either she had changed and everything else had remained the same or that she was real and everything else had changed. On noticing a familiar birthmark, she decided on the latter explanation. Neuropsychological assessment revealed sensory aphasia, color blindness, visual agnosia, and both episodic and semantic memory impairment. MRI and SPECT were unremarkable, and her EEG revealed nonlocalized nonspecific changes. Her overall DES score was 25; however, she scored 69 on the DP/DR subscale. The Capgras syndrome and the symptoms of DP and DR lasted 6 months, gradually resolving.
Case 2. A 48-year-old right-handed woman with no personal or family neuropsychiatric history suffered a brainstem infarct. MRI revealed multiple areas of increased signal affecting the cerebellum, brainstem, posterior part of the thalamus bilaterally, and the left occipital lobe, consistent with ischemic lesions in the vertebrobasilar territory. Six months following the infarct, she developed low mood accompanied by both DP and DR. She referred to herself as the “old” and “new” selves. She described the “new self” as if “part of me is not me” and “something's missing.” She was anxious that the “new self” might take over. She experienced a reality distortion of objects and unfamiliar people in the outside world, which she described as “I know that it's there…but it's not the same,” and “I know that you're there…but you're not.” She also found that she had to concentrate much harder to understand details of conversation and to register details of other objects and people. Paroxetine relieved the depressive ideation but not the DP and DR, which persisted for several months.
Case 3. A 22-year-old right-handed woman with a family history of depression developed partial epilepsy at the age of 17. The seizures were medically intractable, and thus she underwent a left-sided temporal lobectomy at the age of 18. Pathological examination of the resected temporal lobe revealed Rasmussen's encephalitis. Neuroimaging confirmed the complete removal of all the medial temporal structures. Eighteen months later, her seizures recurred, consisting of a “thumping” sensation in her head, along with fear and the feeling of someone being behind her. They were accompanied by symptoms typical of a panic attack, with palpitations, overbreathing, a dry mouth, a sense of the world closing in on her, and a fear of dying. She also experienced marked symptoms of depersonalization and derealization. She felt that she “wasn't there,” that she was not real, and that she was in a dream. She also felt that the surroundings and other people were not real, as though she were watching television. A video-EEG revealed frequent runs of epileptiform activity originating in the left suprasylvian region and rapidly becoming generalized. The EEG changes were accompanied by her symptoms of panic and depersonalization, confirming the ictal nature of the episodes. She scored highly on measures of anxiety and dissociation while having these frequent seizures (Table 3). The episodes ceased on increasing her anticonvulsant medication.
Case 4. A 32-year-old right-handed woman with no personal or family psychiatric history developed medically intractable posttraumatic epilepsy of occipital lobe origin at the age of 25, 9 months after suffering an assault. The seizures consisted of flashing lights in the right hemifield, which at times would generalize into a tonic-clonic seizure. In addition, she experienced complex partial seizures consisting of a feeling of depersonalization during which she lost the “sense of herself” and episodes in which she felt she went “outside herself” and during which she “observed herself.” During these episodes she was unaware of her surroundings, and they were accompanied by automatisms consisting of plucking actions involving her right hand, along with chewing movements. These seizures also had a tendency to generalize. Neuroimaging revealed a meningioma arising from the left temporal lobe. Following the assault she suffered from PTSD and developed a depressive illness, which was treated with cognitive-behavioral therapy. The seizures, and thus the ictal depersonalization, developed several months after the depression had shown some improvement with therapy. Interictally, her overall score on the DES was 4.3, with a DP/DR subscale rating of 3.3. A left temporal lobectomy relieved the episodes of ictal depersonalization and temporal lobe automatisms, but the occipital seizures remained.
DISCUSSION
The literature reveals a large body of published cases of organic DP. However, in many cases it is not clear whether the DP is fleeting, episodic, or chronic as part of a depersonalization disorder. In addition, many cases of DP developed alongside other psychiatric symptoms. However, literature review is complicated by absent or incomplete information. The presence of DP and/or DR as shown in Table 1 was determined from the case descriptions in the individual reports. However, in some cases only terms such as “depersonalization,” “derealization,” “dreamy state,” or “feelings of unreality” were documented, and thus the accuracy of diagnostic classification cannot be assured.
Few of the published reports used rating scales or standardized interviews to assess depersonalization. The DES is used in some of the case series,39,44,52 but this instrument mainly screens for dissociation.41 The six-item DP/DR subscale may be more specific for detecting depersonalization,42,86 but it is rarely reported in the literature. The patient in Case 1 of our series scored much higher on this subscale than on the overall DES (Table 3), suggesting that her dissociation was mostly accounted for by depersonalization. Unfortunately, no cutoff has been established for this subscale. The DES scores for the two cases of ictal depersonalization (Cases 3 and 4) are markedly different. In Case 3 the questionnaire was completed at a time when the patient's EEG studies suggested she was in nonconvulsive status epilepticus, and thus her DES score could be thought of as an ictal assessment. In contrast, in Case 4 the DES was completed during the interictal period, and thus the patient had low scores. Therefore, the DES and its subscales may be useful both for screening and for determining the etiology of depersonalization.
Although DSM-IV cites an equal sex distribution for depersonalization, most studies have found a preponderance of women.9,14,61 Twenty-nine of the 47 previously published cases described female patients; the addition of our 4 reveals that 64.7% of patients with organic depersonalization are female, a figure very similar to the 63% found by Simeon et al.,9 and may reflect the well-known bias of women toward seeking assessment and treatment. The phenomena described seem to be similar to those noted in nonorganic cases, although additional symptoms such as reduplication (Case 1) and personification (Case 2) are rather unusual variants on the depersonalization theme.
Summarizing the nature and site of the brain pathology from the EEG and neuroimaging information available on the 47 published and 4 new cases reveals that only 3 patients (6%) had no documented pathology; 4 were reported as having “diffuse” disease, and in 10 cases there was insufficient information to make any inferences. In remaining 34 cases, the pathology was focal, predominantly affecting the temporal lobe (25 cases). There was no clear evidence of lateralization, with 13 cases being left-sided and 8 right-sided, the rest having bilateral pathology. However, 9 patients had focal pathology affecting extratemporal areas (often in addition to the temporal lobe), of which 7 had right-sided pathology. Moreover, only 1 of the 25 cases of “pure” temporal lobe pathology was right-sided, whereas 11 affected the left, the rest having bilateral disease. Thus, left-sided temporal lobe dysfunction may be a risk factor for the development of depersonalization. Furthermore, other psychiatric disorders, such as depression, have been found to be more common in patients with neurological conditions affecting the left hemisphere, including epilepsy, cerebrovascular disorders, and Parkinson's disease.87 This link suggests that left-sided pathology may facilitate the development of a secondary psychiatric illness.
Epilepsy emerges as the neurological disorder most commonly associated with DP and DR—at least in terms of the number of published articles examining the issue. Migraine appears to be the next. Summarizing the evidence, it seems that focal epilepsy has a stronger link with DP and DR than does primary generalized epilepsy, which Devinsky and colleagues43 found not to be associated. Devinsky et al.43 also found that DP rather than DR was associated with focal epilepsy, whereas the opposite pattern was found by Silberman et al.38 Part of this distinction can be attributed to the baseline rate of the dissociative symptoms in the control groups. It should be noted, too, that people with nonepileptic seizures score highly on DES ratings of DP and DR.44 Hence, although paroxysmal alterations in consciousness and temporal lobe pathology may alone or in combination provide a potent substrate for DP and DR, such dysfunction is clearly neither necessary nor sufficient to cause them.
In the majority of cases, both in the literature review and our four new reports, there was evidence of other psychiatric comorbidity, most commonly depression or anxiety/panic attacks (although in Case 3 the panic attacks were ictal in origin). In most instances it is not possible to ascertain whether the underlying organic condition resulted in both anxiety and depersonalization or whether the DP/DR was instead secondary to a state of high arousal associated with the anxiety. For example, in the patient with a right-sided temporal lobe meningioma reported by Ghadirian et al.,83 DP and DR developed after treatment of her depression and anxiety attacks. Following surgery she had no further episodes of anxiety, DP, or DR, despite ongoing depressive symptoms necessitating therapy. In this case, it is particularly difficult to ascertain whether the DP and DR were secondary to the anxiety disorder or to her meningioma. It has been hypothesized that “depersonalization is a hard-wired vestigial response for dealing with extreme anxiety, combining a state of increased alertness with a profound inhibition of the emotional response system.”88 The mechanism proposed was that in response to high anxiety, the medial prefrontal cortex would inhibit emotional processing on the amygdala and related structures, resulting in a dampening of sympathetic output and reduced emotional experiencing.88 Whether the DP is triggered by an alteration in consciousness/arousal secondary to an organic/toxic state or to a psychological anxiety state remains unclear. Either way, the findings of this review appear to confirm the early belief of Mayer-Gross14 that DP results from a preformed functional response of the brain.
This review brings together many reports of organic depersonalization. Although in the majority of cases there is neurophysiological or radiological evidence of temporal lobe involvement, there is no clear lateralization, although a left-sided preponderance is suggested. The wide range of organic illnesses associated with DP suggests that nonspecific temporal lobe dysfunction along with anxiety may result in the development of depersonalization. However, further studies are needed to determine the relationship.
The stipulation in the DSM-IV that a diagnosis of DP cannot be made if there is a preexisting organic diagnosis results in the exclusion of many patients from various studies,87 thus limiting the available information on the association. The introduction of an organic subtype of depersonalization disorder analogous to “mood disorders due to a general medical condition” in the DSM-IV would fulfill a clinical need and facilitate research in this area. Finally, a more systematic and detailed description of the phenomenology of “organic depersonalization,” coupled with advanced neuroimaging and neurophysiological investigative techniques, will facilitate comparison with “idiopathic” or “functional” depersonalization. This in turn will enable a finer-grained mapping of the cognitive and behavioral subcomponents of depersonalization experiences to their neural substrates.
ACKNOWLEDGMENTS
The authors thank Professor Peter Halligan for bringing Case 2 to their attention. Support for this study came from the Col. W.W. Pilkington Will, Cecil Pilkington, and A.P. Pilkington Pilozzo Charitable Trusts.
 |
 |
 |
1 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994Google Scholar
2 Steinberg M: Derealization: the unreal world, in Handbook for the Assessment of Dissociation: A Clinical Guide, edited by Steinberg M. Washington, DC, American Psychiatric Press, 1995, pp 137-151Google Scholar
3 Roberts WW: Normal and abnormal depersonalization. Journal of Mental Science 1960; 106:478-493Crossref, Medline, Google Scholar
4 Dixon JC: Depersonalization phenomena in a sample population of college students. Br J Psychiatry 1963; 109:371-375Crossref, Medline, Google Scholar
5 Trueman D: Depersonalization in a college sample. J Gen Psychol 1984; 14:980-989Google Scholar
6 Brauer R, Harrow M, Tucker GJ: Depersonalization phenomena in psychiatric patients. Br J Psychiatry 1970; 117:509-515Crossref, Medline, Google Scholar
7 Cassano GB, Petracca A, Perugi G, et al: Derealization and panic attacks: a clinical evaluation on 150 patients with panic disorder/agoraphobia. Compr Psychiatry 1989; 30:5-12Crossref, Medline, Google Scholar
8 Sedman G: An investigation of certain factors concerned in the aetiology of depersonalization. Acta Psychiatr Scand 1972; 48:191-219Crossref, Medline, Google Scholar
9 Simeon D, Gross S, Guralnik O, et al: Feeling unreal: 30 cases of DSM-III-R depersonalization disorder. Am J Psychiatry 1997; 154:1107-1113Crossref, Medline, Google Scholar
10 Torch EM: Review of the relationship between obsession and depersonalization. Acta Psychiatr Scand 1978; 58:191-198Crossref, Medline, Google Scholar
11 Trueman D: Anxiety and depersonalization and derealization experiences. Psychol Rep 1984; 54:91-96Crossref, Medline, Google Scholar
12 World Health Organization: F.48.1 Depersonalization-derealization syndrome, in The ICD-10 Classification of Mental and Behavioral Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, World Health Organization, 1992, pp 171-173Google Scholar
13 Good MI: The concept of an organic dissociative syndrome: what is the evidence? Harv Rev Psychiatry 1993; 1:145-157Crossref, Medline, Google Scholar
14 Mayer-Gross W: On depersonalization. Br J Med Psychol 1935; 15:103-126Crossref, Google Scholar
15 Ackner B: Depersonalization, I: aetiology and phenomenology. Journal of Mental Science 1954; 100:838-853Crossref, Medline, Google Scholar
16 Cohen SI: The pathogenesis of depersonalization: a hypothesis (letter). Br J Psychiatry 1988; 152:578Crossref, Medline, Google Scholar
17 Grigsby J, Kaye K: Incidence and correlates of depersonalization following head trauma. Brain Inj 1993; 7:507-513Crossref, Medline, Google Scholar
18 Noyes R, Kletti R: Depersonalization in the face of life threatening danger: a description. Psychiatry 1976; 39:19-27Crossref, Medline, Google Scholar
19 Shilony E, Grossman FK: Depersonalization as a defense mechanism in survivors of trauma. J Trauma Stress 1993; 6:119-128Crossref, Google Scholar
20 Thivierge J, Julien Y: Case report: auditory evoked potentials and psychiatry. Can J Psychiatry 1988; 33:552-554Crossref, Medline, Google Scholar
21 Cantagallo A, Grassi L, Della Sala S: Dissociative disorder after traumatic brain injury. Brain Inj 1999; 13:219-228Crossref, Medline, Google Scholar
22 Grigsby JP: Depersonalization following minor closed head injury. International Journal of Clinical Neuropsychology 1986; 8:65-69Google Scholar
23 Gronwall D: Rehabilitation programmes for patients with mild head injury: components, problems and evaluation. J Head Trauma Rehabil 1986; 1:53-62Crossref, Google Scholar
24 Jackson JH, Colman WS: Case of epilepsy with tasting movements and “dreamy state”: very small patch of softening in the left uncinate gyrus. Brain 1898; 21:580-590Crossref, Google Scholar
25 Langs G, Fabisch H, Fabisch K, et al: Can cannabis trigger recurrent panic attacks in susceptible patients? Eur Psychiatry 1997; 12:415-419Crossref, Medline, Google Scholar
26 Greenberg DB, Hochberg FH, Murray GB: The theme of death in complex partial seizures. Am J Psychiatry 1984; 141:1587-1589Crossref, Medline, Google Scholar
27 Kanemoto K: Periictal Capgras syndrome after clustered ictal fear: depth-electroencephalogram study. Epilepsia 1997; 38:847-850Crossref, Medline, Google Scholar
28 Davison K: Episodic depersonalization. Br J Psychiatry 1964; 110:505-513Crossref, Medline, Google Scholar
29 Stern TA, Murray GB: Complex partial seizures presenting as a psychiatric illness. J Nerv Ment Dis 1984; 172:625-627Crossref, Medline, Google Scholar
30 Edlund MJ, Swann AC, Clothier J: Patients with panic attacks and abnormal EEG results. Am J Psychiatry 1987; 144:508-509Crossref, Medline, Google Scholar
31 Dantendorfer K, Amering M, Baischer W, et al: Is there a pathophysiological and therapeutic link between panic disorder and epilepsy? Acta Psychiatr Scand 1995; 91:430-432Crossref, Medline, Google Scholar
32 Standage KF, Fenton GW: Psychiatric symptom profiles of patients with epilepsy: a controlled investigation. Psychol Med 1975; 5:152-160Crossref, Medline, Google Scholar
33 Mullan S, Penfield W: Illusions of comparative interpretation and emotion. Archives of Neurology and Psychiatry 1959; 81:269-284Crossref, Google Scholar
34 Harper M, Roth M: Temporal lobe epilepsy and the phobic anxiety-depersonalization syndrome, part 1: a comparative study. Compr Psychiatry 1962; 3:129-151Crossref, Medline, Google Scholar
35 Kenna JC, Sedman G: Depersonalization in temporal lobe epilepsy and the organic psychoses. Br J Psychiatry 1965; 111:293-299Crossref, Medline, Google Scholar
36 Smirnov VYa: Paroxysmal psychopathological symptoms in patients with brain tumors in the right and left temporal lobes. Neurosci Behav Physiol 1977; 8:86-89Crossref, Medline, Google Scholar
37 Schenk L, Bear D: Multiple personality and related dissociative phenomena in patients with temporal lobe epilepsy. Am J Psychiatry 1981; 138:1311-1316Crossref, Medline, Google Scholar
38 Silberman EK, Post RM, Nurnberger J, et al: Transient sensory, cognitive and affective phenomena in affective illness: a comparison with complex partial epilepsy. Br J Psychiatry 1985; 146:81-89Crossref, Medline, Google Scholar
39 Devinsky O, Putnam F, Grafman J, et al: Dissociative states and epilepsy. Neurology 1989; 39:835-840Crossref, Medline, Google Scholar
40 Bernstein EM, Putnam FW: Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis 1986; 174:727-735Crossref, Medline, Google Scholar
41 Steinberg M, Rounsaville B, Cicchetti D: Detection of dissociative disorders in psychiatric patients by a screening instrument and a structured diagnostic interview. Am J Psychiatry 1991; 148:1050-1054Crossref, Medline, Google Scholar
42 Carlson EB, Putnam FW, Ross CA, et al: Factor analysis of the Dissociative Experiences Scale: a multicenter study, in Proceedings of the Eighth International Conference on Multiple Personality and Dissociative States, edited by Braun BG, Carlson EB. Chicago, Rush, 1991Google Scholar
43 Devinsky O, Feldmann E, Bromfield E, et al: Structured interview for partial seizures: clinical phenomenology and diagnosis. Journal of Epilepsy 1991; 4:107-116Crossref, Google Scholar
44 Alper K, Devinsky O, Perrine K, et al: Dissociation in epilepsy and conversion nonepileptic seizures. Epilepsia 1997; 38:991-997Crossref, Medline, Google Scholar
45 Kuyk J, Spinhoven P, Van Emde Boas W, et al: Dissociation in temporal lobe epilepsy and pseudo-epileptic seizure patients. J Nerv Ment Dis 1999; 187:713-720Crossref, Medline, Google Scholar
46 Persinger MA, Makarec K: Complex partial epileptic signs as a continuum from normals to epileptics: normative data and clinical populations. J Clin Psychol 1993; 49:33-45Crossref, Medline, Google Scholar
47 Toni C, Cassano GB, Perugi G, et al: Psychosensorial and related phenomena in panic disorder and in temporal lobe epilepsy. Compr Psychiatry 1996; 37:125-133Crossref, Medline, Google Scholar
48 Dening TR, Berrios GE: Autoscopic phenomena. Br J Psychiatry 1994; 165:808-817Crossref, Medline, Google Scholar
49 Kamiya S, Okamoto S: Double consciousness in epileptics: a clinical picture and minor hemispheric specialization, in Advances in Epileptology: XIIIth Epilepsy International Symposium, edited by Akimoto H, Kazamatsuri H, Seino M, Ward A. New York, Raven, 1982, pp 397-401Google Scholar
50 Putnam FW, Guroff JJ, Silberman EK, et al: The clinical phenomenology of multiple personality disorder: review of 100 recent cases. J Clin Psychiatry 1986; 47:285-293Medline, Google Scholar
51 Coons PM, Bowman ES, Milstein V: Multiple personality disorder: a clinical investigation of 50 cases. J Nerv Ment Dis 1988; 176:519-527Crossref, Medline, Google Scholar
52 Ross CA, Heber S, Anderson G, et al: Differentiating multiple personality disorder and complex partial seizures. Gen Hosp Psychiatry 1989; 11:54-58Crossref, Medline, Google Scholar
53 Mesulam M-M: Dissociative states with abnormal temporal lobe EEG. Multiple personality and the illusion of possession. Arch Neurol 1981; 38:176-181Crossref, Medline, Google Scholar
54 Benson DF, Miller BL, Signer SF: Dual personality associated with epilepsy. Arch Neurol 1986; 43:471-474Crossref, Medline, Google Scholar
55 Drake ME: Epilepsy and multiple personality: clinical and EEG findings in 15 cases (abstract). Epilepsia 1986; 27:635Google Scholar
56 Coons PM, Milstein V, Marley C: EEG studies of two multiple personalities and a control. Arch Gen Psychiatry 1982; 39:823-825Crossref, Medline, Google Scholar
57 Cocores JA, Bender AL, McBride E: Multiple personality, seizure disorder, and the electroencephalogram. J Nerv Ment Dis 1984; 172:436-438Crossref, Medline, Google Scholar
58 Penfield W, Perot P: The brain's record of auditory and visual experience: a final summary and discussion. Brain 1963; 86:595-696Crossref, Medline, Google Scholar
59 Halgren E, Walter RD, Cherlow DG, et al: Mental phenomena evoked by electrical stimulation of the human hippocampal formation and amygdala. Brain 1978; 101:83-117Crossref, Medline, Google Scholar
60 Gloor P, Olivier A, Quesney LF, et al: The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Ann Neurol 1982; 12:129-144Crossref, Medline, Google Scholar
61 Shorvon HJ, Hill J, Burkitt E, et al: The depersonalization syndrome. Proc R Soc Med 1946; 39:779-792Medline, Google Scholar
62 Kew J, Wright A, Halligan PW: Somesthetic aura: the experience of “Alice in Wonderland.” Lancet 1998; 351:1934Crossref, Medline, Google Scholar
63 Ogunyemi AO: Migraine with prolonged aura: correlation of clinical and EEG features. Behavioural Neurology 1995; 8:109-114Crossref, Medline, Google Scholar
64 Cutler B, Reed J: Multiple personality: a single case study with a 15-year follow-up. Psychol Med 1975; 5:18-26Crossref, Medline, Google Scholar
65 Lippman CW: Certain hallucinations peculiar to migraine. J Nerv Ment Dis 1952; 116:346-351Crossref, Medline, Google Scholar
66 Lippman CW: Hallucinations of physical duality in migraine. J Nerv Ment Dis 1953; 117:345-350Crossref, Medline, Google Scholar
67 Todd J: The syndrome of Alice in Wonderland. CMAJ 1955; 73:701-704Medline, Google Scholar
68 Golden GS: The Alice in Wonderland syndrome in juvenile migraine. Pediatrics 1979; 63:517-519Medline, Google Scholar
69 Copperman SM: “Alice in Wonderland” syndrome as a presenting symptom of infectious mononucleosis in children. Clin Pediatr 1977; 16:143-146Crossref, Medline, Google Scholar
70 Sanguinetti G, Crovato F, De Marchi R, et al: “Alice in Wonderland” syndrome in a patient with infectious mononucleosis (case report). J Infect Dis 1983; 147:782Crossref, Medline, Google Scholar
71 Eshel GM, Eyov A, Lahat E, et al: Alice in Wonderland syndrome, a manifestation of acute Epstein-Barr virus infection (brief report). Pediatr Infect Dis J 1987; 6:68Crossref, Medline, Google Scholar
72 Lahat E, Eshel G, Arlazoroff A: “Alice in Wonderland” syndrome and infectious mononucleosis in children (letter). J Neurol Neurosurg Psychiatry 1990; 53:1104Crossref, Medline, Google Scholar
73 Cinbis M, Aysun S: Alice in Wonderland syndrome as an initial manifestation of Epstein-Barr virus infection (case report). Br J Ophthalmol 1992; 76:316Crossref, Medline, Google Scholar
74 Wang S-M, Liu C-C, Chen Y-J, et al: Alice in Wonderland syndrome caused by Coxsackievirus B1. Pediatr Infect Dis J 1996; 15:470-471Crossref, Medline, Google Scholar
75 Pelletier G, Legendre-Roberge J, Boileau B, et al: Case study: dreamy state and temporal lobe dysfunction in a migrainous adolescent. J Am Acad Child Adolesc Psychiatry 1995; 34:297-301Crossref, Medline, Google Scholar
76 Schilder P: The Image and Appearance of the Human Body. New York, International Universities Press, 1950Google Scholar
77 Rigatelli M, Casolari L, Bergamini G, et al: Psychosomatic study of 60 patients with vertigo. Psychother Psychosom 1984; 41:91-99Crossref, Medline, Google Scholar
78 Grigsby JP, Johnston CL: Depersonalization, vertigo and Ménière's disease. Psychol Rep 1989; 64:527-534Crossref, Medline, Google Scholar
79 Fewtrell WD, O'Connor KP: Dizziness and depersonalization. Adv Behav Res Ther 1988; 10:201-218Crossref, Google Scholar
80 Lilja Å, Salford LG: Early mental changes in patients with astrocytomas with special reference to anxiety and epilepsy. Psychopathology 1997; 30:316-323Crossref, Medline, Google Scholar
81 Malamud N: Psychiatric disorder with intracranial tumors of limbic system. Arch Neurol 1967; 17:113-123Crossref, Medline, Google Scholar
82 Ardila A, Rosseli M: Temporal lobe involvement in Capgras syndrome. Int J Neurosci 1988; 43:219-224Crossref, Medline, Google Scholar
83 Ghadirian AM, Gauthier S, Bertrand S: Anxiety attacks in a patient with a right temporal lobe meningioma. J Clin Psychiatry 1986; 47:270-271Medline, Google Scholar
84 Wall M, Tuchman M, Mielke D: Panic attacks and temporal lobe seizures associated with a right temporal lobe arteriovenous malformation: case report. J Clin Psychiatry 1985; 46:143-145Medline, Google Scholar
85 Morioka H, Nagatomo I, Horikiri Y, et al: A case of pontine infarction with depersonalization. IMJ Ill Med J 1997; 4:133-134Google Scholar
86 Simeon D, Guralnik O, Gross S, et al: The detection and measurement of depersonalization disorders. J Nerv Ment Dis 1998; 186:536-542Crossref, Medline, Google Scholar
87 Robertson MM: Depression in neurological disorders, in Depression and Physical Illness, edited by Robertson MM, Katona CLE. New York, Wiley, 1997, pp 305-339Google Scholar
88 Sierra M, Berrios GE: Depersonalization: neurobiological perspectives. Biol Psychiatry 1998; 44:898-908Crossref, Medline, Google Scholar
89 Hollander E, Carrasco JL, Mullen LS, et al: Left hemispheric activation in depersonalization disorder: a case report. Biol Psychiatry 1992; 31:1157-1162Crossref, Medline, Google Scholar



