Predicting Treatment Response in Obsessive-Compulsive Disorder
“Possession” by evil spirits was once the diagnosis for those who were having obsessive ruminations about religion, sex, or other subjects that were intrusive or distressing. Although the concept of “possession” was dismissed long ago, modern medicine still does not have a complete understanding of what is now termed “obsessive-compulsive disorder” (OCD). This anxiety disorder is characterized by intrusive reoccurring thoughts or images that have such themes as danger, contamination, religion, sex, or symmetry. These thoughts are distressing to the sufferer and are recognized as irrational. To relieve anxiety associated with the thoughts, the obsessor often engages in compulsions or ritualistic actions. These include counting, checking, hand washing, or repetition of words, phrases, or other actions. Many good reviews of this disorder have been published to update clinicians on the latest theories of pathologic development, diagnosis, prognosis, and treatment strategies.1–3 OCD has a worldwide prevalence of 2% to 3% and a mean age of onset in the twenties to thirties, although it often arises in childhood.4 It is a chronic disorder with many comorbid conditions. For example, two-thirds of OCD patients have major depressive disorder.
Many studies have been done to identify the neuroanatomical substrate of OCD. Much of this work has involved neuroimaging. Initial structural studies with computed tomography (CT) and magnetic resonance imaging (MRI) revealed a variety of volumetric or asymmetric changes in cortical and subcortical structures of OCD patients. The consistent theme was abnormalities in the circuits that include the orbitofrontal and anterior cingulate cortices, basal ganglia, and thalamus. Additional work with functional imaging has allowed a further refinement in the proposed abnormal circuitry, including increased activity in the excitatory or direct pathways as compared with the indirect or inhibitory pathways through the basal ganglia. Saxena et al.5 have published an excellent review describing these pathways and the neuroimaging studies that led to the current neuroanatomical theory of OCD. They not only review the circuitry, but also update the four areas of imaging studies in OCD: studies comparing OCD patients and control subjects; studies of patients before and after treatment; studies provoking symptoms; and studies of cognitive activation. Theories to explain these circuit abnormalities include absence of normal developmental “pruning” during neuronal maturation, streptococcal infection, hypoxia to the striosomes of the striatum, and genetic abnormalities in enzyme and monoamine functions.5,6
Treatment is presently unpredictable, with most patients achieving only a partial response. The current standard of care is to offer behavioral psychotherapy and medication. The medications of choice are selective serotonin reuptake inhibitors (SSRIs), which allow 65% to 70% of patients to attain a moderate (30% to 60%) improvement in symptoms.7 Psychosurgery (cingulotomy or capsulotomy) is done very infrequently to help the most severe nonresponders to all other therapeutic options. This treatment also provides only modest improvements.3 It would clearly be immensely valuable to separate patients into more homogeneous groups in order to target treatment strategies. The literature relevant to prediction of treatment response is both promising and sparse. The varied approaches can be grouped into those focused on pre-treatment symptoms, electrophysiological parameters, and functional brain imaging.
PREDICTION BASED ON SYMPTOMS: DIMENSIONAL MODEL OF OCD
An obvious hypothesis, given the extensive range of symptoms that may be present in OCD, is that the predominant symptoms displayed can be used to group patients into treatment categories. Most studies have approached this by categorizing patients into subgroups based on presence/absence of specific OCD symptoms, such as compulsions or cleaning rituals. This approach has not been particularly successful (see reviews8,9). A more promising approach applies factor analysis techniques to identify symptoms that are highly intercorrelated, thus collapsing many symptoms into a few symptom dimensions.8–10 In the initial study, the many items in the Yale-Brown Obsessive Compulsive Scale symptom checklist were subsumed into three major dimensions: aggressive/sexual/religious; contamination; and symmetry/hoarding.10 The symmetry/hoarding dimension correlated with comorbid Tourette's syndrome or chronic tic disorder, suggesting that this subgroup would benefit from neuroleptic medication. In the larger follow-up study, the number of dimensions was expanded to five: symmetry/ordering; hoarding; contamination/cleaning; aggressive/checking; and sexual/religious obsessions.9 Not surprisingly, overall severity of symptoms prior to treatment was related to post-treatment severity of symptoms. In addition, higher scores on the hoarding dimension correlated with poorer response to SSRIs.
PREDICTION BASED ON ELECTROPHYSIOLOGICAL MEASURES
Both quantitative electroencephalography (qEEG) and event-related potential (ERP) studies have been done in the context of predicting treatment response in OCD.
In qEEG analyses, a mathematical method (the Fast Fourier Transform) is used to convert a block of EEG into a power spectrum so that concrete measures can be made. The power spectrum is split into standard frequency bands (delta, 1.5–3.5 Hz; theta, 3.5–7.5 Hz; alpha, 7.5–12.5 Hz; beta, 12.5–25 Hz) prior to measurement of absolute and relative power, mean frequency, and inter/ intrahemispheric coherence/symmetry. In a pilot study, baseline (pre-treatment) measures were compared for responders and nonresponders to medication (clomipramine or fluoxetine).11 Responders showed increased power in the alpha and beta bands, particularly in frontal regions. Nonresponders showed decreased power in the delta band, particularly in the posterior regions. In a follow-up study, cluster analysis was used to identify subgroups.12 Decreased power in the delta band was present in both subgroups of patients. Increased power in the theta band, particularly in anterior regions, defined patient group 1. Increased power in the alpha and beta bands, particularly in the anterior and central regions, defined patient group 2. Group 1 contained mostly nonresponders (71%), group 2 mostly responders (88%). These results were replicated in a larger study from the same group.13 The authors note that SSRIs have been shown to decrease alpha activity. Thus, SSRIs may help normalize brain activity in responsive patients. On the basis of these findings, they suggest that OCD may include pathophysiologically different subgroups with a similar clinical expression.
Event-related potentials (ERPs) are the summed electrical activity recorded from scalp electrodes following a stimulus presentation. A variety of situations or tasks are used in this type of study, and the ERP has a characteristic shape for each. A very simple task commonly used requires monitoring of a series of sounds (e.g., tones, words) and responding in some manner (e.g., pushing a button, keeping count) whenever a designated different sound is presented (“oddball” task). The different, rarely occurring, sound is the target. Early portions of the waveform are related to initial sensory reception and analysis of the stimulus. The subsequent N2 and P3 peaks relate to stimulus evaluation and perceptual synthesis.14,15 In one set of studies, the latencies of the N2 and P3 peaks were shortened in patients who later responded well to SSRIs.14,15 The amplitude of the N2 component may also be predictive of SSRI responsiveness, but in a complex manner. When simple sound stimuli (tones) were used, an increased N2 amplitude correlated with SSRI responsiveness.16 When complex sound stimuli (words) were used, decreased N2 amplitude correlated with SSRI responsiveness.14,15 Thus, both accelerated processing and abnormal stimulus evaluation in this task may identify OCD patients likely to respond well to SSRIs. The authors note that these findings are consistent with the cortical hyperarousal model of OCD. These are promising results, but relatively few patients were studied, and the robustness and generalizability of the findings have not yet been demonstrated.
PREDICTION BASED ON FUNCTIONAL BRAIN IMAGING
Two types of functional brain imaging have been used to study prediction of treatment response in OCD patients: positron emission tomography (PET) and single-photon emission computed tomography (SPECT). Functional imaging uses radioactive tracers followed by imaging to measure brain activity. All of the PET studies mentioned below used glucose utilization ([18F]fluorodeoxyglucose; FDG-PET) as their tool for examining brain function. The published SPECT study involved measurements of regional cerebral blood flow (rCBF) using [99mTc]HMPAO. In comparison with SPECT, PET has better spatial resolution, but it is more expensive and not widely available. Although together these studies are very few in number, complex, and expensive to undertake, the concepts studied are exciting and the results are encouraging.
PET Imaging
One of the first studies to look for predictors of treatment response used FDG-PET scans in 18 adults with childhood-onset OCD.17 Patients who had pre-treatment lower metabolic rates in the right orbitofrontal and right anterior cingulate cortices responded better initially to clomipramine (2 months of treatment). However, these measures were not predictive of good therapeutic response at 1 year.18 Interestingly, good therapeutic response at 1 year correlated with pre-treatment normalized rate of metabolism (region of interest divided by whole hemisphere) in the left caudate. However, on later follow-up scans, responders and nonresponders had similar left caudate metabolic rates. The authors noted uncertainty as to how this finding should be interpreted.
Brody et al.19 performed FDG-PET before and after treatment in 27 patients diagnosed with OCD. Eighteen of the patients had chosen behavioral therapy and 9 had chosen fluoxetine treatment. Results were surprising. In the behavioral therapy group, patients with higher pre-treatment normalized metabolism in left orbitofrontal cortex responded better. In the medication group, patients with lower pre-treatment normalized metabolism in left orbitofrontal cortex responded better. The authors propose that the behavioral therapy results may be related to the orbitofrontal cortex function of mediating responses to situations where the “affective value” changes. Behavioral therapy targets that assignment of value. Results for the fluoxetine group were similar to previous work in which higher orbitofrontal metabolism (although on the right rather than left) was associated with more severe illness and less robust response to medication.18 One possible source of bias in this study is that the patients self-selected their therapy. A patient's biology may be associated with choice of treatment.
A follow-up study by the same group20 examined changes in brain metabolism before and after paroxetine treatment. A secondary finding in this study is important to prediction of treatment response. Patients with lower normalized metabolism bilaterally in orbitofrontal cortex prior to treatment responded better to paroxetine. More specifically, the left posterior medial orbitofrontal cortex had the “strongest relationship” with better response. As noted by the authors, further work with large groups and placebo controls is needed. This group has also studied SSRI-refractory patients treated with adjunctive risperidone (S. Saxena, personal communication). Risperidone responders had higher pre-treatment glucose metabolism in right posteromedial orbitofrontal cortex and bilateral thalamus (Figure 1). Lower pre-treatment glucose metabolism in left parietal and bilateral dorsolateral prefrontal cortices, however, was also associated with better response. Thus, there may be different, and perhaps opposite, neurochemistries among patients who respond to atypical antipsychotics.
Another SSRI, fluvoxamine, has been used in a PET study to predict treatment response. Results are in press, but the authors refer to the outcome in another published article. In this work, an inverse correlation was found between rCBF in the orbitofrontal cortex and treatment response, as well as a direct relationship between rCBF in the posterior cingulate cortex and treatment response.21
One other area of PET prediction of treatment response has had a first examination. It is prediction of response to anterior cingulotomy. Rauch et al.21 retrospectively examined the presurgical PET scans of 11 patients who underwent anterior cingulotomies for refractory severe OCD. Higher presurgical regional cerebral metabolic rates for glucose utilization within posterior cingulate cortex were associated with better postoperative improvement in symptoms (Figure 2). The authors suggest that this finding may be related to a direct influence of the posterior cingulate on orbitofrontal cortex and caudate, in addition to its influence on the anterior cingulate. As noted with other pre-treatment studies, this one had limitations including small numbers, scans done during a resting state, and reliance on a linear model of evaluation that did not account for variables such as comorbid conditions and medication effects.
SPECT Imaging
In a recent double-blind trial of sertraline versus desipramine for patients with OCD and comorbid major depression, 11 of 16 participants were classified as OCD treatment responders.22 In a retrospective examination of pre-treatment scans, responders had higher rCBF in the orbitofrontal cortex (left>right), cingulate cortex, and basal ganglia than did nonresponders, irrespective of the medication used (Figure 3). The authors propose that “changes in cerebral activity correspond with changes in psychopathology rather than reflecting response-independent effects of treatment modalities.” The study, however, had small numbers and all patients had comorbid major depression.
Although the data are not available in a PubMed-indexed journal, a recent SPECT study of 37 OCD patients was reported at the Seventh World Congress of Biological Psychiatry.23 In this report, the presenter noted that the SSRI responders had greater pre-treatment rCBF in the right caudate and lower rCBF in the anterior cingulate with symptom provocation.
CONCLUSION
Unlike many other neuropsychiatric disorders, OCD has reasonably well established circuitry. However, a substantial subset of OCD patients remain treatment refractory. As with most psychiatric conditions, there are no robust predictive tests to guide sequencing of treatment trials. Although traditionally considered a single diagnostic entity, OCD is now thought to be an etiologically and phenotypically diverse condition.8 The heterogeneous nature of this severely disabling condition creates a substantial challenge. Pre-treatment indicators of likely treatment response would therefore be immensely valuable.
Previous studies of treatment prediction have not separated patients according to subtypes of OCD. Many have not included patients with comorbid disorders, although these are common. The meaning and interpretation of these studies might be different if more homogeneous subgroups of patients were compared. The great variety of approaches that has been used creates another challenge, making comparisons across studies difficult. Most studies have used relatively small groups and most have not been independently replicated. All studies to date were retrospective rather than prospective. Functional imaging studies have not all measured the same areas of brain, further complicating comparisons across studies.
Even with these problems, the reported results suggest that particular patterns of pre-treatment regional brain activity in OCD differentially predict treatment response (to behavioral therapy, SSRIs, adjunctive atypical antipsychotics, and neurosurgery). Prediction of treatment response based on pre-treatment brain activity could become increasingly important in the future for understanding the mechanisms of treatment response. This opens the door to an exciting new prospect of being able to use functional testing to help guide treatment.
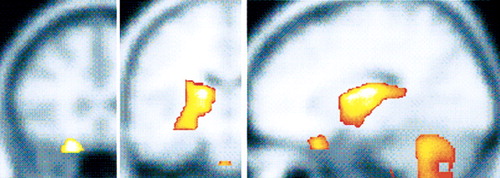
Figure 1. Retrospective analysis of pre-treatment PET regional cerebral glucose uptake (rCMRglu) scans in OCD patients. Response of OCD to paroxetine was associated with higher pre-treatment rCMRglu in the right caudate, whereas response to adjunctive risperidone in refractory patients was associated with higher pre-treatment rCMRglu in right orbitofrontal cortex and bilateral thalamus, as indicated in color (light yellow is highest level of significance) on coronal (left, middle) and sagittal (right) magnetic resonance images.
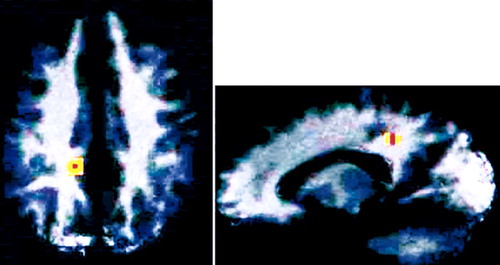
Figure 2. Retrospective analysis of preoperative PET regional cerebral glucose uptake (rCMRglu) scans in OCD patients prior to cingulotomy. A correlation analysis was performed between preoperative rCMRglu and improvement in postoperative Yale-Brown Obsessive Compulsive Scale score. The only area that was statistically significant was the posterior cingulate, in which higher preoperative rCMRglu was associated with greater postoperative improvement. This area is indicated on axial (left) and sagittal (right) magnetic resonance images (reprinted with permission from Rauch et al.21).
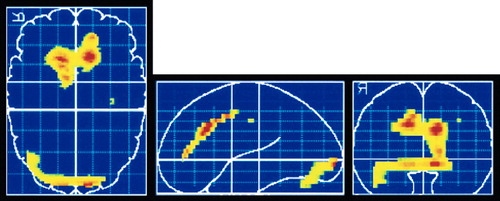
Figure 3. Retrospective analysis of pre-treatment SPECT regional cerebral blood flow (rCBF) scans in OCD patients. Higher rCBF in anterior cingulate prior to treatment was found in medication responders, as indicated in color (red indicates highest level of difference) on axial (left), sagittal (middle), and coronal (right) brain templates (reprinted with permission from Hoehn-Saric et al.22).
1 Allen A, Hollander E (eds): Obsessive-Compulsive Spectrum Disorders (special issue). Psychiatr Clin North Am 2000; 23(3):xv,469-687Google Scholar
2 Neel JL, Stevens VM, Stewart JE: Obsessive-compulsive disorder: identification, neurobiology, and treatment. J Am Osteopath Assoc 2002; 102:81-86Medline, Google Scholar
3 Jenike MA: An update on obsessive-compulsive disorder. Bull Menninger Clin 2001; 65:4-25Crossref, Medline, Google Scholar
4 Horwath E, Weissman MM: The epidemiology and cross-national presentation of obsessive-compulsive disorder. Psychiatr Clin North Am 2000; 23:493-507Crossref, Medline, Google Scholar
5 Saxena S, Bota RG, Brody AL: Brain-behavior relationships in obsessive-compulsive disorder. Semin Clin Neuropsychiatry 2001; 6:82-101Crossref, Medline, Google Scholar
6 Stein DJ: Advances in the neurobiology of obsessive-compulsive disorder: implications for conceptualizing putative obsessive-compulsive and spectrum disorders. Psychiatr Clin North Am 2000; 23:545-562Crossref, Medline, Google Scholar
7 Hollander E, Kaplan A, Allen A, et al: Pharmacotherapy for obsessive-compulsive disorder. Psychiatr Clin North Am 2000; 23:643-656Crossref, Medline, Google Scholar
8 Leckman JF, Grice DE, Boardman J, et al: Symptoms of obsessive-compulsive disorder. Am J Psychiatry 1997; 154:911-917Crossref, Medline, Google Scholar
9 Mataix-Cols D, Rauch SL, Manzo PA et al: Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatry 1999; 156:1409-1416Medline, Google Scholar
10 Baer L: Factor analysis of symptom subtypes of obsessive compulsive disorder and their relation to personality and tic disorders. J Clin Psychiatry 1994; 55(suppl):18-23Google Scholar
11 Prichep LS, Mas F, John ER, et al: Neurometric subtyping of obsessive compulsive disorders in psychiatry: a world perspective, in Proceedings of the VIII World Congress of Psychiatry, Athens, October 12-19, 1989. Edited by Stefanis CN, Rabavilas AD, Soldatos CR. New York, Elsevier Science, 1989, pp 557-562Google Scholar
12 Mas F, Prichep LS, John ER, et al: Neurometric quantitative electroencephalogram subtyping of obsessive compulsive disorders, in Imaging of the Brain in Psychiatry and Related Fields, edited by Maurer K. Berlin-Heidelberg, Springer-Verlag, 1993, pp 277-280Google Scholar
13 Prichep LS, Mas F, Hollander E, et al: Quantitative electroencephalographic subtyping of obsessive-compulsive disorder. Psychiatry Research: Neuroimaging 1993; 50:25-32Crossref, Medline, Google Scholar
14 Morault PM, Bourgeois M, Laville J, et al: Psychophysiological and clinical value of event-related potentials in obsessive-compulsive disorder. Biol Psychiatry 1997; 42:46-56Crossref, Medline, Google Scholar
15 Morault P, Guillem F, Bourgeois M, et al: Improvement predictors in obsessive-compulsive disorder: an event-related potential study. Psychiatry Res 1998; 81:87-96Crossref, Medline, Google Scholar
16 Towey J, Bruder G, Tenke C, et al: Event-related potential and clinical correlates of neurodysfunction in obsessive-compulsive disorder. Psychiatry Res 1993; 49:167-181Crossref, Medline, Google Scholar
17 Swedo SE, Schapiro MB, Grady CL, et al: Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry 1989; 46:518-523Crossref, Medline, Google Scholar
18 Swedo SE, Pietrini P, Leonard HL, et al: Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder: revisualization during pharmacotherapy. Arch Gen Psychiatry 1992; 49:690-694Crossref, Medline, Google Scholar
19 Brody AL, Saxena S, Schwartz JM, et al: FDG-PET predictors of response to behavioral therapy and pharmacotherapy in obsessive compulsive disorder. Psychiatry Research: Neuroimaging 1998; 84:1-6Crossref, Medline, Google Scholar
20 Saxena S, Brody AL, Maidment KM, et al: Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology 1999; 21:683-693Crossref, Medline, Google Scholar
21 Rauch SL, Dougherty DD, Cosgrove GR, et al: Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for obsessive compulsive disorder. Biol Psychiatry 2001; 50:659-667Crossref, Medline, Google Scholar
22 Hoehn-Saric R, Schlaepfer TE, Greenberg BD, et al: Cerebral blood flow in obsessive-compulsive patients with major depression: effect of treatment with sertraline or desipramine on treatment responders and non-responders. Psychiatry Research: Neuroimaging 2001; 108:89-100Crossref, Medline, Google Scholar
23 Sherman C: Brain studies predict OCD treatment response. Clinical Psychiatry News, Sept 2001, p 12. www.imng.com/ IMNG/psyche/Google Scholar



